Monkeypox Case Lands in Dallas

The Centers for Disease Control and Prevention (CDC) and the Texas Department of State Health Services (DSHS) confirmed on July 15, 2021, a case of human monkeypox in a U.S. resident who recently traveled from Nigeria to the USA.
The patient is currently hospitalized in Dallas, Texas.
The CDC confirmed it is working with the airline and state and local health officials to contact airline passengers and others who may have been in contact with the patient during two flights:
- Lagos, Nigeria, to Atlanta on July 8th, with arrival on July 9th; and
- Atlanta to Dallas on July 9th.
Since travelers on these flights and in airports were required to wear face masks due to the ongoing COVID-19 pandemic, therefore, it’s believed the risk of the spread of monkeypox via respiratory droplets to others on the planes and in the airports is low, says the CDC.
Monkeypox is in the same family of viruses as smallpox but causes a milder infection.
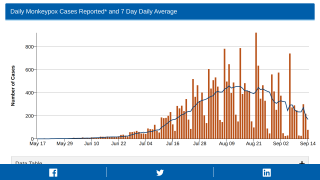
Monkeypox is a rare but potentially severe viral illness that typically begins with flu-like illness and swelling of the lymph nodes and progresses to a widespread rash on the face and body. Most infections last 2-4 weeks.
In this case, laboratory testing at CDC showed the patient is infected with a strain of monkeypox most commonly seen in parts of West Africa, including Nigeria. Infections with this strain of monkeypox are fatal in about 1% of people.
However, rates can be higher in people who have weakened immune systems.
Before the current case, at least six reported monkeypox cases in travelers returning from Nigeria (including patients in the United Kingdom, Israel, and Singapore). This case is not related to any of these previous cases. In the United Kingdom, several additional monkeypox cases occurred in people who had contact with patients.
Monkeypox also caused a large outbreak in the USA in 2003 after the virus spread from imported African rodents to pet prairie dogs.
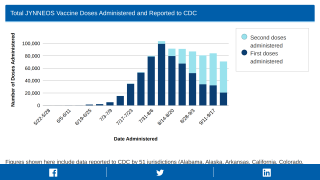
There is one preventive vaccine available in the USA.
The U.S. Biomedical Advanced Research and Development Authority recently exercised the final $12 million option for JYNNEOS, an innovative combination of Smallpox and Monkeypox vaccine.
JYNNEOS is the only U.S. Food and Drug Administration-approved non-replicating smallpox and monkeypox vaccine for preventing disease in adults 18 years of age and older.
CDC is also running trials in the Democratic Republic of Congo to assess whether the smallpox vaccine Jynneos may help protect healthcare workers from contracting undiagnosed monkeypox infections from their patients.
At CDC labs in Atlanta, GA, scientists have also provided laboratory testing, including specialized tests to identify people who may have had monkeypox and recovered, sequencing to trace outbreaks, and phylogenetics to determine if clusters of cases were related. In addition, CDC continues to train Nigerian partners to collect wildlife to test for which animals carry the virus in nature, helping to improve the country’s ability to track monkeypox cases in people and interview community members about their interactions with local wildlife.
For more information about monkeypox, visit CDC: Monkeypox.
PrecisionVaccinations publishes research-based vaccine news.
Our Trust Standards: Medical Advisory Committee