Who Says Staff Doesn't Need Monkeypox Vaccinations
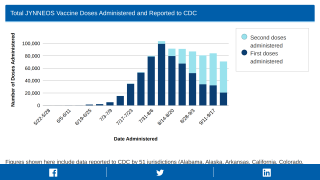
The U.S. Department of Health and Human Services' Administration for Strategic Preparedness and Response (ASPR) recently confirmed it is making a total of 1.1 million vials of Bavarian Nordic's Jynneos® vaccine available to jurisdictions in the USA to support monkeypox response efforts.
As of September 14, 2022, ASPR had released 807,093 vials to all jurisdictions for free.
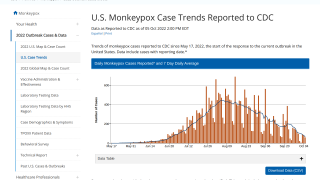
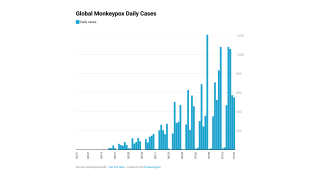
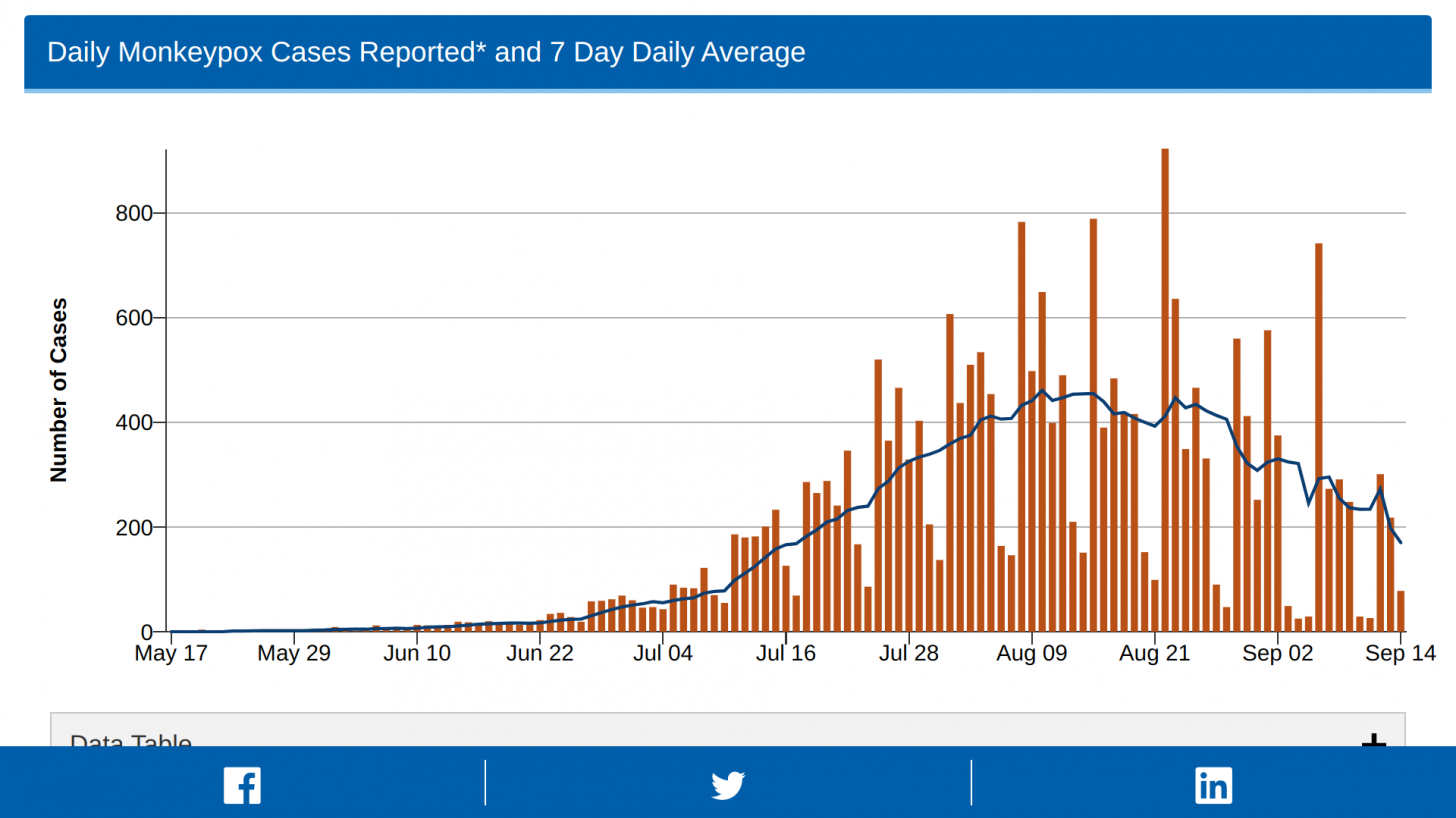
These Jynneos vaccines support the 23,893 confirmed monkeypox/orthopoxvirus cases in the USA as of September 19, 2022.
While the supply of Jynneos vaccines is improving, various studies have indicated one group of people may not need this preventive vaccine.
The Colorado Department of Public Health and Environment (CDPHE) recently evaluated healthcare provider (HCP) exposures and personal protective equipment (PPE) use in healthcare settings during the care of patients who subsequently received a diagnosis of Orthopoxvirus infection or monkeypox.
From May to July 31, 2022, a total of 313 HCP interacted with patients with subsequently diagnosed monkeypox infections while wearing various combinations of PPE:
- 23% wore all recommended PPE during their exposures.
- 28% of exposed HCP were considered to have had high- or intermediate-risk exposures and were therefore eligible to receive postexposure prophylaxis with the JYNNEOS vaccine.
- Among those, 48% (12% of all exposed HCP) received the Jynneos vaccine.
- PPE use varied by facility type.
The good news reported by the U.S. CDC on September 16, 2022, was that 'no HCP developed a monkeypox infection during the 21 days after exposure.'
The CDC concluded that 'this study illustrated that the risk for HCP acquiring monkeypox after exposure to patients with monkeypox was very low despite incomplete adherence to recommended PPE.'
And in the United Kingdom, the Modified Vaccinia Ankara (Jynneos, Imvanex) vaccine is not being offered to healthcare staff who work in non-specialist wards or clinics, even those in frontline services and Accident and Emergency.
The UKHSA stated on September 6, 2022, that 'staff is at very low risk of exposure.'
And the monkeypox vaccine is also not being offered to men who have sex with men, who have fewer partners and have a much lower chance of coming into close contact with a case.
However, the U.K says 'individuals with a community exposure should be offered post-exposure vaccination if they are in risk categories.'
Furthermore, the debate on Jynneos's efficiency against the multiple strains of monkeypox continues.
Recent data from India indicates three versions of monkeypox are currently circulating globally.
The Indian Council of Medical Research- NIV Pune analyzed the complete genome sequences of monkeypox cases and found three sub-clusters among A.2 lineage.
The first cluster, Kerala (n5) and Delhi (n2), aligned with the USA-2022 ON674051.1; while the second of Delhi (n3), aligned with USA-2022 ON675438.1, and a third cluster consists of the U.K., U.S., and Thailand.
The WHO's recent briefing concluded on September 7, 2022, that there is 'no peer-reviewed clinical data regarding current monkeypox vaccine efficacy. But expects data to be available in October 2022.'
MonkeypoxToday.com publishes breaking news and research regarding the ongoing outbreak.
PrecisionVaccinations publishes fact-checked, research-based vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee
- U.S. Monkeypox National Vaccine Strategy
- Health Care Personnel Exposures to Subsequently Laboratory-Confirmed Monkeypox Patients
- UKHSA Guidance Monkeypox: waiting for your vaccination
- WHO press conference on COVID-19, monkeypox and other global health issues
- Monkeypox vaccine research
- UK monkeypox guidance