HIV and Monkeypox Combo Remain Global Concern

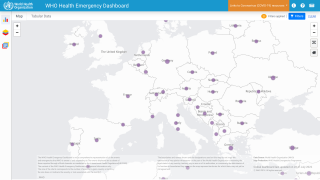
The WHO Emergency Committee recently acknowledged that some progress had been made in the global response to the multi-country outbreak of monkeypox since the last meeting.
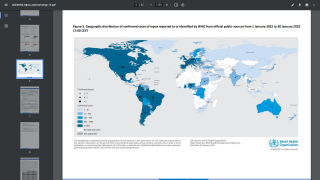
The Committee held the consensus view on November 1, 2022, that the event continues to meet the criteria for a Public Health Emergency of International Concern and highlights the primary reasons for ongoing concern.
These include an emerging potential for more significant health impact in vulnerable populations, continuing risk of stigma and discrimination, and other outbreak gaps.
The Committee noted the epidemiological concomitance of monkeypox, HIV, and other Sexually Transmitted Infections.
And expressed concern about the more frequent severe outcomes and deaths in people living with HIV who are immunocompromised and/or not receiving antiretroviral treatment, especially in underserved and low-resource settings.
Many low-income settings have inadequate diagnostic capacity.
However, little clinical information is available on when the monkeypox virus (MPX) is actually spread.
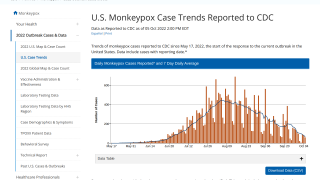
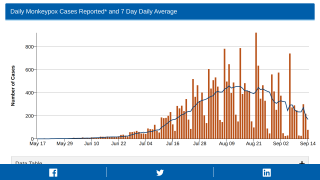
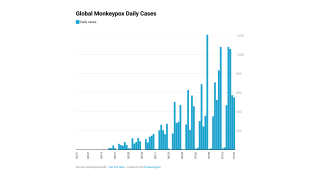
Although case numbers are declining, the increased international transmission would facilitate infection importation and might drive outbreaks even if vaccination in local networks limits transmission.
For example, the monkeypox epidemic in the U.K. has been largely based in dense social networks with high contact rates.
Remarkably, transmission has been largely clustered around a subset of gay, bisexual, and other men who have sex with men.
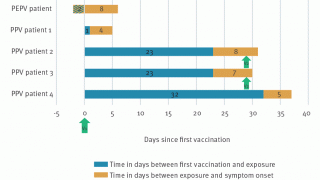
A recent U.K.-based study found the mean incubation period and mean serial interval range from 6.6 to 9.2 days and 7.4 to 12.3 days, respectively when adjusted for bias.
And the peer-review journal The Lancet published the findings from a study on October 7, 2022, that found of 209 patients for whom HIV status was known, 92 (44%) men had HIV infection.
And of 219 patients for whom data were available, 216 (99%) reported sexual or close intimate contact in the 21 days before symptom onset.
And the U.S. CDC published on October 31, 2022, considerations for the care of people with HIV, including prevention and treatment of monkeypox.
The CDC's Key Points are as follows:
- People with HIV-associated immunosuppression or not virologically suppressed can be at increased risk of severe disease with monkeypox.
- Post-exposure prophylaxis is available for people exposed to MPX, and antiviral treatment is available for people with MPV Vaccination with JYNNEOS is considered safe for people with HIV, and antiviral medicines for monkeypox have few interactions with antiretroviral therapy.
- The use of tecovirimat (TPOXX) should be considered in people with HIV, particularly those with immunosuppression.
- The addition of other therapeutics (cidofovir, brincidofovir, VIGIV) may be considered for those not improving or those progressing while on tecovirimat.
- Screening for STIs should be considered for persons evaluated for monkeypox infection, with appropriate care offered to those with positive test results.
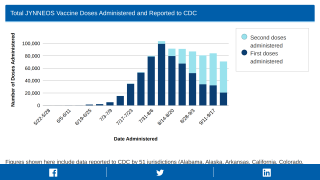
While there is a U.S. FDA-approved vaccine for monkeypox, two-dose JYNNEOS® (IMVANEX®), there is not one for HIV.
However, several HIV vaccine candidates are conducting clinical studies.
Vax-Before-Travel publishes fact-checked, research-based travel vaccine news.
Our Trust Standards: Medical Advisory Committee
- Third WHO meeting of the International Health Regulations - monkeypox
- CDC - Monkeypox Vaccine Administration in the U.S.
- BMJ - Transmission dynamics of monkeypox in the U.K.
- CDC - Clinical Considerations for Treatment and Prophylaxis of Monkeypox
- The Lancet - clinical characteristics of patients with monkeypox