Cardiac Risk Counseling Advised Before Monkeypox Vaccination

A recent non-peer-reviewed study based on Kaiser Permanente Northwest (KPNW) data intended to document any cardiac events associated with Bavarian Nordic's two-dose JYNNEOS® vaccine.
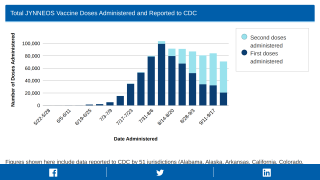
Since May 2022, about 600,000 people have received one or more vaccine doses in the U.S.
And New York City began offering people second doses of JYNNEOS in September 2022.
However, this study published on November 14, 2022, found that all identified cardiac events had alternative explanations, and no hospitalizations or serious adverse outcomes were attributed to monkeypox vaccination.
This research was initiated because of the U.S. FDA safety data for JYNNEOS that notes potential cardiac adverse events of particular interest following vaccination.
And the U.S. CDC's Advisory Committee on Immunization Practices (ACIP) stated in 2022 that people with underlying heart disease or three or more major cardiac risk factors should be counseled about the theoretical risk for myopericarditis following vaccination with JYNNEOS.
A few people who have gotten the smallpox vaccine (ACAM2000) have developed heart inflammation (myocarditis), inflammation of the lining of the heart (pericarditis), or a combination of both (myopericarditis), wrote the CDC in 2017.
These researchers conducted a retrospective cohort study using KPNW databases reviewing ICD-10-CM diagnosis codes.
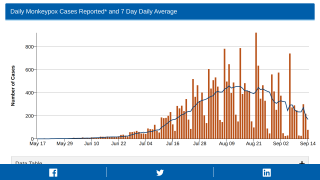
There were 2,126 KPNW patients aged 12 or older who received 3,235 JYNNEOS vaccine doses between July 14, 2022, and October 10, 2022.
After physician adjudication, there were ten confirmed cardiac AESI for an incidence of 3.1 per 1000 doses given with (95% CI, 1.5 to 5.7).
Of these ten events, none could be attributed directly to the JYNNEOS vaccination.
In summary, these researchers wrote, 'This retrospective cohort study of JYNNEOS vaccination for prevention of monkeypox identified ten cardiac events that all had alternative explanations, and no hospitalizations or serious adverse outcomes were attributed to vaccination.'
Furthermore, 'this initial study provides timely information for the clinician counseling their patient with underlying cardiac risk factors on the low observed risk of cardiac events with JYNNEOS vaccination during the 2022 monkeypox public health emergency.'
The study authors declared no competing interest, and this study did not receive any outside funding.
The JYNNEOS (MVA-BN®) vaccine is marketed under the brand names IMVANEX® in the European Union and IMVAMUNE® in Canada.
Pablo Sanchez, MD, led the U.S. CDC's ACIP Monkeypox Vaccine presentation on October 20, 2022.
Severe adverse reactions known for replicating vaccinia virus strains, such as myocarditis, encephalitis, generalized vaccinia, or eczema vaccinatum, were not observed during the JYNNEOS clinical development program.
And JYNNEOS is contraindicated in persons with a serious allergy to any vaccine component, says the CDC. The vaccine contains trace amounts of chicken protein, benzonase and gentamicin, and ciprofloxacin.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee