Innovative Dengue Vaccine Readies in Europe

After five years of clinical research, a new dengue vaccine may soon become available throughout Europe.
The European Medicines Agency (EMA) human medicines committee adopted a positive opinion for QDENGA® (TAK-003) Dengue Tetravalent Vaccine (live, attenuated) on October 14, 2022.
Japan-based Takeda's QDENGA has been endorsed for the prevention of dengue disease caused by any serotype in individuals four years of age and older in Europe and dengue-endemic countries participating in the parallel EU-M4all procedure.
The final step in the approval path is gaining Marketing Authorization.
QDENGA is the only dengue vaccine currently approved for use regardless of previous dengue exposure and without needing pre-vaccination testing.
"We are one step closer towards the approval of a dengue vaccine that could benefit many of the millions of individuals around the world exposed to Dengue. This is a major moment for the global health community, European countries, and the dengue-endemic countries that participated in the EU-M4all procedure," said Gary Dubin, M.D., president of the Global Vaccine Business Unit at Takeda, in a related press release.
"We have been working for many years to help improve how dengue can be prevented."
"Our efforts to provide a new option for dengue prevention support Takeda's overall goal to provide long-term societal value to the people we serve."
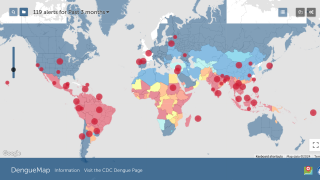
The incidence of Dengue has grown dramatically around the world in recent decades, causing an estimated 390 million infections and 500,000 hospitalizations annually. Dengue viruses are spread to people through the bite of an infected Aedes mosquito, says the WHO.
Dengue is the second most diagnosed cause of fever in travelers returning to Europe from endemic countries.
Its presence is far-reaching in endemic countries across the Americas, South-East Asia, and Western Pacific regions. In addition, it is growing in non-endemic areas in continental Europe, including France, Italy, Germany, Spain, and the United States.
As of October 17, 2022, the U.S. CDC had confirmed 616 dengue cases in the U.S. and 209 dengue cases in U.S. Territories.
And during the summer of 2022, the Florida Department of Health in Miami-Dade County issued a mosquito-borne illness advisory. There were 18 locally acquired dengue cases in Miami-Dade County.
"The global health community has been eager for a dengue vaccine that is accessible without the barrier of pre-vaccination testing," said Dr. Ooi Eng Eong, Professor of Emerging Infectious Diseases at Duke-NUS Medical School in Singapore.
"The robust clinical data provided by Takeda shows that its dengue vaccine has the potential to help prevent dengue cases and hospitalizations. So today, we are closer to helping improve dengue prevention and reducing the burden of disease on countries, communities, and health systems."
According to the U.S. CDC's Laura Adams, DVM, MPH, and Liliana Sánchez-González, MD, MPH, presentation on September 29, 2022, Dengue is endemic in six U.S. territories and freely associated states.
Previously, the U.S. FDA approved the Dengvaxia vaccine for limited use in May 2019 for people with previous dengue infection and living in dengue-endemic areas.
Dengue outbreaks and vaccine news are posted at Vax-Before-Travel.com/Dengue.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee