FDA Committee Debates RSV Vaccine(s) Risk/Benefit

New Vaccines and Related Biological Products Advisory Committee (VRBPAC) meetings have been scheduled this week to consider the safety of two Respiratory Syncytial Virus (RSV) vaccine candidates for seniors.
This U.S. Food and Drug Administration (FDA) vaccine committee includes industry leaders offering expert opinions in response to strategic questions.
The FDA's 179th meeting agenda and various digital presentations on February 28 and March 1, 2023, highlight these questions:
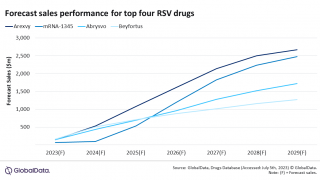
- Are the available data adequate to support the safety of Pfizer's ABRYSVO (RSVPreF) when administered to individuals 60 years of age and older to prevent lower respiratory tract disease (LRTD) caused by RSV?
- Are the available data adequate to support the safety of GSK's AREXVY (RSVPreF3+AS01E) when administered to individuals 60 years of age and older to prevent LRTD caused by RSV?
On February 28, 2023, under Topic #1, the VRBPAC will meet in an open session to discuss and make recommendations on the safety and effectiveness of ABRYSVO (RSV Vaccine), manufactured by Pfizer Inc., with a requested indication, in Biologics License Application (BLA) 125769, for active immunization for the prevention of acute respiratory disease and LRTD caused by RSV in adults 60 years of age and older.
On March 1, 2023, under Topic #2, the vaccine committee will discuss and make recommendations on the safety and effectiveness of AREXVY (RSV Vaccine, Recombinant, Adjuvanted), manufactured by GSK, with a requested indication, in BLA 125775, for active immunization for the prevention of LRTD caused by RSV-A and RSV-B subtypes in adults 60 years of age and older.
As of February 27, 2023, the FDA has not authorized any RSV vaccine candidate.
The FDA uses committees and panels to obtain independent expert advice on scientific, technical, and policy matters but is not bound to follow committee votes. VRBPAC meetings are open to the public, with selected consumer participation.
These VRBPAC meetings follow last week's U.S. Centers for Disease Control and Prevention (CDC) vaccine committee (ACIP) discussions focused on RSV vaccinations of infants and seniors and potential modifications to using RSV monoclonal antibody passive immunization.
Our Trust Standards: Medical Advisory Committee