Are There Enough Monkeypox Vaccines for Everyone?

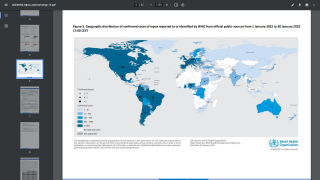
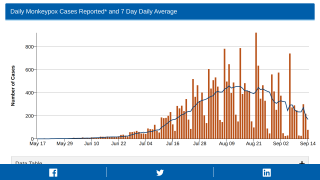
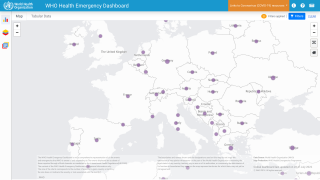
The goal of any preventive vaccination program is to limit the spread of disease in the community. However, with monkeypox infections occurring in about twenty countries, anyone who has been in close contact with an infected person could qualify for vaccination.
"I want to emphasize that we're in the early days of this (monkeypox) response. Unfortunately, additional cases will likely be reported in the U.S.," Jennifer McQuiston, a deputy director of the CDC's National Center for Emerging and Zoonotic Diseases, reported RollCall on May 23, 2022.
While the global monkeypox outbreak has reached about twenty countries, many have asked if the U.S. FDA-approved vaccines will become available.
Since both monkeypox and smallpox belong to the poxvirus family, genus Orthopoxvirus, two vaccines may become available in the USA.
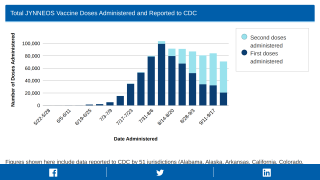
However, the recently approved Jynneos® vaccine is in limited supply everywhere.
In England, the UKHSA recently announced it had already distributed about 1,000 Jynneos (IMVANEX®) doses to monkeypox patients’ close contacts, with only 3,500 remaining in inventory.
As of May 23, 2022, the UKHSA has confirmed 56 monkeypox patients.
And in the USA, there are requests to release the Jynneos vaccine from the U.S. Strategic National Stockpile (SNS) for the general public.
But the SNS only has a few thousand doses available.
"Right now, we are hoping to maximize vaccine distribution to those we know would benefit from it," said Captain Jennifer McQuiston, deputy director of the CDC's High Consequence Pathogens and Pathology Division, reported Reuters.
The two-dose Jynneos vaccine was developed in partnership with the U.S. Government to ensure adults can be protected from smallpox/monkeypox, including those with weakened immune systems or at high risk of adverse reactions to traditional smallpox vaccines based on replicating vaccinia virus strains.
The good news is the U.S. government has already ordered additional Jynneos vaccines.
On May 18, 2022, the government exercised contract options (119 million doses) to purchase a freeze-dried version of the Jynneos vaccine.
While Bavarian Nordic A/S ramps up Jynneos production, an old smallpox vaccine may be an immediate substitute.
The other FDA-approved vaccine is Emergent BioSolutions, Inc.’s ACAM2000®.
Approved in 2007, ACAM2000 is a live vaccinia virus, a replication-competent vaccine, to protect against smallpox disease. This vaccine does not contain variola and cannot cause smallpox.
It consists of a live, infectious vaccinia virus that can be transmitted from the vaccine recipient to unvaccinated persons who have close contact with the inoculation site or exudate from the site.
The vaccinia virus may cause side effects like rash, fever, and body aches.
This older vaccine has proven to be effective in preventing smallpox over many years. Most U.S. military personnel are given ACAM2000 while in ‘boot camp.’
The good news is the SNS has more than 100 million doses of ACAM2000 in stock.
And on January 9, 2022, Emergent BioSolutions confirmed that ACAM2000 vaccine deliveries are expected to continue under the existing contract with the U.S. at unit volume levels consistent with 2021 deliveries.
But it is not monkeypox approved by the FDA.
To solve the potential monkeypox vaccine shortfall, a leading mRNA vaccine producer may enter the monkeypox market segment.
On May 23, 2022, Moderna Inc. Tweeted: as monkeypox is of global public health importance identified by the WHO, we are investigating potential monkeypox vaccines at a preclinical level.
PrecisionVaccinations publishes fact-checked research-based vaccine news.
Our Trust Standards: Medical Advisory Committee