9,437 Evaluable People Enrolled in Late-Stage Lyme Disease Vaccine Study
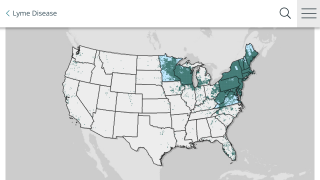
With the winter months ahead, most hikers are not focused on catching Lyme disease. However, once the snow melts, millions of people will once again not have access to a vaccine.
There are currently no approved human vaccines for Lyme disease.
To address this significant health risk, Pfizer Inc. and Valneva SE today announced that they have completed recruitment for the Phase 3 clinical trial Vaccine Against Lyme for Outdoor Recreationists (VALOR) for Lyme disease vaccine candidate VLA15.
The VALOR trial, initiated in August 2022, has enrolled 9,437 participants five years of age and older at sites where Lyme disease is highly endemic across the U.S., Europe, and Canada.
As part of the primary vaccination series, participants receive three doses of VLA15 or a saline placebo (1:1 ratio) within the first year and one booster dose approximately one year after completion of the primary immunization.
The trial builds on previous positive Phase 1 and 2 trial results and includes adult and pediatric participants to confirm the efficacy, safety, lot consistency, and immunogenicity of VLA15.
"Lyme disease is the most prevalent vector-borne infectious disease in the United States and Europe, can sometimes even lead to long-lasting consequences," said Annaliesa Anderson, Ph.D., Senior Vice President and Head Vaccine Research and Development, Pfizer, in a press release on December 4, 2023.
"If approved, a vaccine could prevent the disease and ease the burden of acute, severe, and sometimes persistent consequences in adults and children."
"We look forward to progressing the trial with the goal of submitting a Biologics License Application to the U.S. Food and Drug Administration and Marketing Authorization Application to the European Medicines Agency in 2026, subject to positive data."
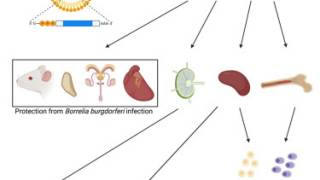
VLA15 is an alum-adjuvanted formulation administered intramuscularly and has demonstrated a strong immune response and a satisfactory safety profile in pre-clinical and clinical trials.
This investigational multivalent protein subunit vaccine uses an established mechanism of action for a Lyme disease vaccine that targets the outer surface protein A (OspA) of Borrelia burgdorferi, the bacteria that cause Lyme disease.
OspA is a surface protein the bacteria expresses when present in a tick. Blocking OspA inhibits the bacterium's ability to leave the tick and infect humans.
The vaccine candidate covers the six most common OspA serotypes expressed by the Borrelia burgdorferi sensu lato species prevalent in North America and Europe.
Our Trust Standards: Medical Advisory Committee