Is the United States Prepared for Mpox Clade I Arrival

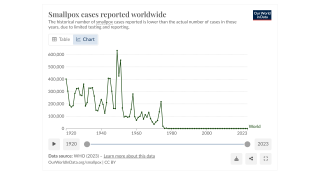
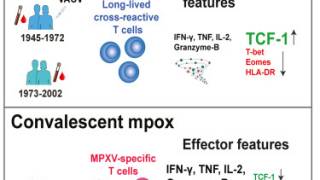
Compared with the clade II mpox virus (MPXV) outbreak in adults that started in May 2022, the ongoing clade I MPXV outbreak in Africa is causing severe illness and higher mortality.
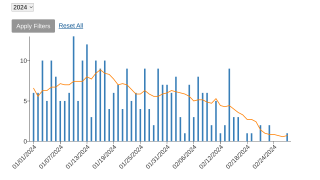
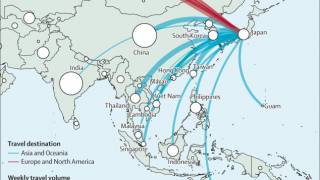
The U.S. Centers for Disease Control and Prevention (CDC) reported on May 16, 2024, that the increasing number of reported suspected clade I mpox cases in the Democratic Republic of the Congo (DRC) poses a global threat for potential spread.
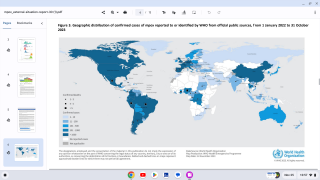
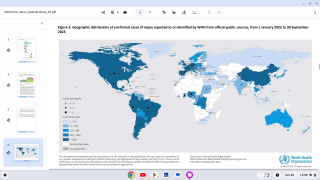
From January 2023 to April 14, 2024, the DRC reported multiple provincial-level outbreaks, comprising 19,919 suspected clade I mpox cases and 975 (4.9%) deaths. A significant percentage of these confirmations were in young people.
To date, no cases of clade I mpox have been detected outside of countries in Central Africa where the virus is endemic.
The CDC says clinicians and public health practitioners should be alert for possible cases in travelers from DRC and request clade-specific testing. Furthermore, appropriate medical treatment and vaccination will be essential to prevent the spread of clade I in the U.S.
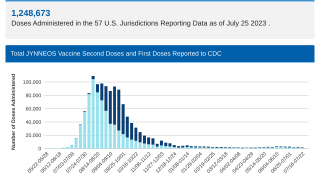
Unfortunately, only 23% of persons in the United States at risk for clade II MPXV infection have completed the 2-dose JYNNEOS® vaccination series.
The CDC encourages persons at risk for clade II MPXV infection to be vaccinated with two doses of the third-generation non-replicating JYNNEOS vaccine.
After April 2024, the cost of the JYNNEOS vaccine may be covered by some health insurance plans, and the vaccine may be available at pharmacies.
As of May 2024, Bavarian Nordic, the producer of JYNNEOS, has not published vaccine efficacy data against preventing clade I MPXV infection.
Our Trust Standards: Medical Advisory Committee