Novel Oral Type-2 Poliovirus Vaccines Reported Safe

Two novel live attenuated, monovalent oral type-2 poliovirus vaccine (OPV2) candidates were found to be safe and immunogenic in adults immunized by the Sabin inactivated polio vaccine (IPV).
Sabin IPV is a liquid trivalent vaccine produced from Sabin poliovirus type 1, 2, and 3 strains grown on Vero cells.
According to a study published in The Lancet, vaccine virus was detected in the stools of 100 percent of the participants receiving vaccine candidate #1, and in 87 percent of participants receiving vaccine candidate #2.
And, vaccine poliovirus shedding stopped at a median of 23 days after vaccine candidate #1 administration and 12 days after candidate #2 administration.
Both novel OPV2 candidates were immunogenic and increased the median blood titer of serum neutralizing antibodies; all participants were seroprotected after vaccination.
Both vaccine candidates had acceptable tolerability, and no serious adverse events occurred during the study.
These OPV2 vaccine candidates were developed to minimize the risk for outbreaks from vaccine-derived polioviruses. And, they are more genetically stable than existing OPVs, and they have a lower risk for reversion to neurovirulence.
This double-blind, single-center phase 1 clinical trial represents the first human-testing of these 2 candidates.
To minimize the risk of environmental release, 48 participants in this double-blind, single-center phase trial, NCT03430349 had been previously vaccinated with IPV and were isolated in a containment facility built at the University of Antwerp Hospital in Antwerp, Belgium.
However, severe events were reported in 40 percent of the participants receiving candidate #1 and 60 percent of participants receiving candidate #2. These events were primarily increased blood creatinine phosphokinase but were not accompanied by clinical signs or symptoms.
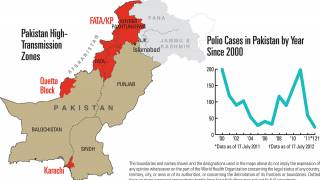
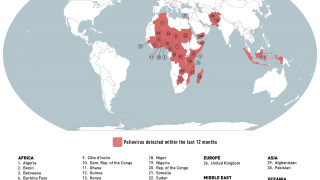
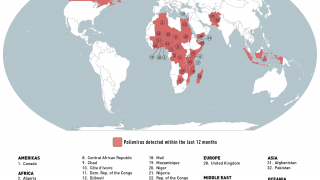
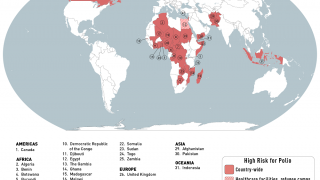
This new study is important since several countries are reporting new polio cases in 2019.
Previously, the world was on the threshold of eradicating poliovirus.
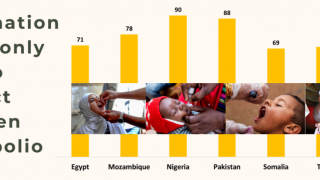
The global strategy to eradicate polio is based on preventing infection by immunizing every child to stop transmission and ultimately make the world polio-free.
Eradication of wild type-2 polioviruses was confirmed in September 2015, leading the WHO Strategic Advisory Group of Experts to recommend global cessation of use of vaccines containing live Sabin type-2 poliovirus.
By May 2016, trivalent oral poliovirus vaccines (OPV) were replaced with bivalent OPV that only contained poliovirus type 1 and type 3, and they were supplemented with at least one dose of trivalent inactivated poliovirus vaccine (IPV) in a global effort.
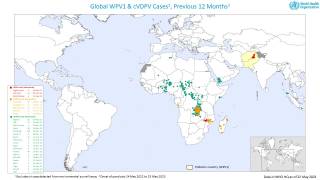
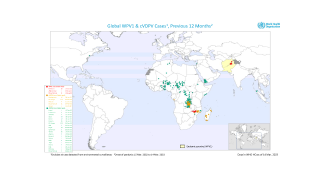
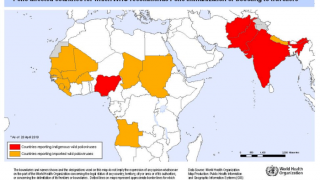
Since May 2016, distinct circulating vaccine-derived type-2 poliovirus (cVDPV2) outbreaks have occurred in seven countries.
cVDPVs have been designated a Public Health Emergency of International Concern, for which the only effective control is the use of stockpiled monovalent OPV2.
The use of this vaccine carries the inherent risk of seeding new cVDPVs, particularly with waning mucosal immunity of the population following OPV2 cessation.
IPV use cannot generate cVDPVs but these vaccines do not induce primary intestinal mucosal immunity, so IPV use is ineffective in interrupting transmission in settings where transmission is predominantly via the fecal-oral route.
Therefore novel OPV2 candidates have been developed with improved genetic stability, which decreases the likelihood of reversion to neurovirulence, thereby minimizing the risk of generating new cVDPVs.
Recent polio news:
- Polio Vaccinations Receive $100 Million Dollar Boost
- Wild Poliovirus Eradication Can Be Achieved
- Hajj Vaccination Requirements Updated for 2019
In summary, these researchers say ‘the novel clinical evidence generated by this clinical trial, can be used to accelerate the next phase of vaccine clinical development.
Furthermore, this new information can inform global policy-making regarding the replacement of the global stockpiles of monovalent Sabin OPV2 with novel OPV2, in response to ongoing and future outbreaks of circulating vaccine-derived type-2 poliovirus.
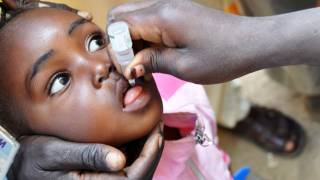
Poliomyelitis is a crippling and potentially fatal infectious disease. There is no cure.
Polio spreads from person to person invading the brain and spinal cord and causing paralysis, says the CDC.
For additional information on countries with polio circulation and vaccine recommendations, consult the travel notices on the CDC Travelers’ Health website.
Pre-trip, polio vaccine counseling appointments can be scheduled at Vax-Before-Travel.
Our Trust Standards: Medical Advisory Committee