Penn Medicine Successfully Tested Immunotherapy Approach Enabling T Cells to Infiltrate Tumors
A therapeutic vaccine was reported in a new study to boost antibodies and T cells, helping them infiltrate tumors and fight off human papillomavirus (HPV)-related head and neck cancer.
Cancer researchers from the Abramson Cancer Center of the University of Pennsylvania tested the immunotherapy approach in patients with advanced head and neck squamous cell carcinoma (HNSCCa) and found 86 percent showed elevated T cell activity.
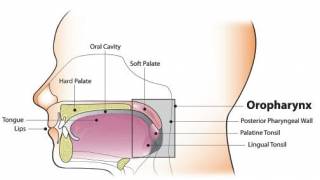
HNSCCa is cancer that develops in the mucous membranes of the mouth and throat, says Cancer.gov.
Additionally, this was the first study to show that the therapeutic vaccine can help immune cells infiltrate tumors, said these researchers in a press release.
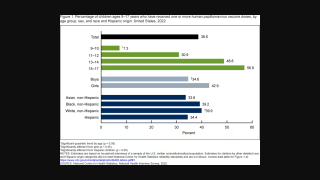
The Centers for Disease Control and Prevention (CDC) says almost all sexually active adults will contract HPV at some point in their lifetime.
And, the CDC estimates 70 percent of all head and neck cancers in the United States are now HPV-related.
While there are many types of HPV, the HPV 16 and 18 subtypes are most commonly associated with cancer. Many patients with this type of HNSCCa cancer have good outcomes from treatment that includes surgery or chemotherapy and radiation.
For patients who don’t respond to treatment or who develop metastatic disease, anti-PD-1 therapy is approved, but only helps about 15 percent of patients.
“We wanted to know if this vaccine can boost the immune systems of patients with HPV-related head and neck cancer, potentially opening the door for better response rates to other existing therapies, and our findings show that we can,” said the study’s lead author Charu Aggarwal, MD, MPH, an assistant professor of Hematology-Oncology in Penn’s Perelman School of Medicine.
The vaccine used in this clinical trial is different from common preventative HPV vaccines, which can prevent but cannot treat cancer.
The vaccine in this study, known as MEDI0457, is a DNA vaccine that can have a therapeutic benefit.
MEDI0457’s two main components are VGX-3100, a DNA plasmid containing modified sequences for E6 and E7, and INO-9012, a DNA plasmid containing the immune activator IL-12.
Plasmids are pieces of circular DNA that contain the sequences necessary to promote the expression of a certain gene.
These Penn researchers gave 4 doses of MEDI0457 to 21 patients separated into two different groups.
Dr. Aggarwal said, “These findings open the door for utilizing targeted immunotherapy approaches against specific cancer-causing targets like HPV.”
“These are very important and unique results, and the next step is already underway looking at combining this vaccine with other immune therapies,” said Roger B. Cohen, MD, a professor of Hematology-Oncology at Penn and Associate Director of Clinical Research in the Abramson Cancer Center.
“We think our results from the single patient who is currently disease free suggest that the clinical impact of a combined vaccine and PD-1 antibody approach may be profound if it can be confirmed in ongoing and planned clinical trials.”
Dr. Cohen helped design the study published in Clinical Cancer Research and was a co-author.
This study was supported by the National Cancer Institute (P30-CA-016520-38), as well as MedImmune and Innovio Pharmaceuticals, Inc., who supplied the vaccine and supported the immune analyses.
Our Trust Standards: Medical Advisory Committee