HIV Vaccine Research Rekindles in 2024
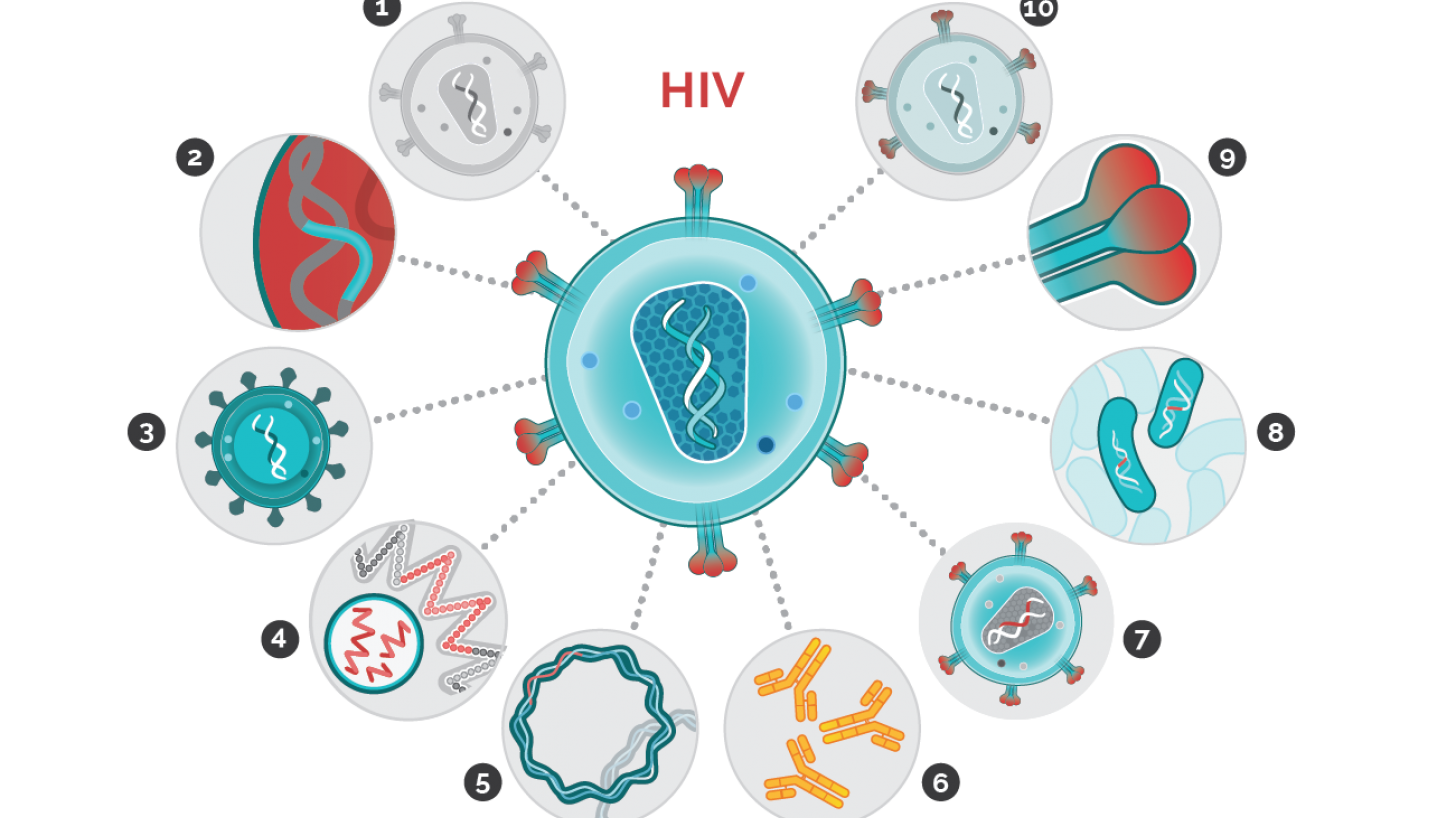
Following the announcement in December 2023 that the PrEPVacc study team was discontinuing vaccinations following a review that determined a minimal chance the vaccine candidates being tested could stop human immunodeficiency virus (HIV) acquisition, many were disappointed.
With an estimated 1.3 million people worldwide becoming infected with HIV annually, there is a tremendous unmet need for a preventive vaccine.
However, recent news indicates other HIV vaccine candidates are moving forward to address this market need in 2024.
Uvax Bio, LLC, a spin-off vaccine company from the highly regarded Scripps Research, is utilizing rational and computational biology to design and deliver novel 1c-SApNP® protein-based vaccines.
With great anticipation, Uvax announced on January 30, 2024, that the first participant was dosed in a Phase 1 clinical trial in Australia evaluating the Company's HIV-1 vaccine candidates, UVAX-1107 and UVAX-1197.
The two antigens differ insofar as UVAX-1197 retains the virus' wild-type glycan shield, while UVAX-1107 has a portion of the glycan shield removed through glycan trimming (GT).
GT is an enzymatic removal of glycans to allow better access to the conserved neutralizing epitopes on HIV-1 Env.
The UFO trimer, 1c-SApNP platform, and glycan trimming strategy were developed by Dr. Jiang Zhu, Associate Professor, Department of Integrative Structural and Computational Biology at Scripps Research.
The study's first-in-human clinical trial primary endpoints will measure the safety and immunogenicity of UVAX-1107 and UVAX-1197 after the primary and boosting dose series are evaluated in parallel arms.
The study will also determine whether either vaccine or combination produces the optimal immunological response.
In the January 30th press release, Uvax Bio stated that it expects to report topline data from the Phase 1 trial in the fourth quarter of 2024.
Ji Li, Ph.D., Uvax Bio CEO, commented, "UVAX-1107 and UVAX-1197 represent an advancement in HIV vaccine technology with industry-recognized innovations in our antigen design and delivery. I am proud of the Uvax Bio team for bringing our promising HIV-1 vaccine candidates into human testing, a significant milestone for the Company."
In a preclinical toxicology study, UVAX-1107 and UVAX-1197 combined with adjuvant were shown to be safe with no serious adverse events, consistent with previously approved protein-based vaccines.
Additionally, a second preclinical immunogenicity study demonstrated immunization with UVAX-1107 and UVAX-1197, which elicited robust neutralizing antibody responses against the vaccine-matched virus in 99% of the animals.
Furthermore, preliminary screening assays demonstrated appreciable neutralization in serum when tested against a panel of primary HIV-1 isolates.
The HIV Vaccine Trials Network says there are many types of vaccines. Still, all generally work by teaching the immune system to recognize and fight back against a disease-causing microorganism.
Some vaccines are designed to help the immune system prevent an infection and are called preventive vaccines. Others are therapeutic vaccines designed to help people who already have a disease to clear the infection.
As of February 2, 2024, there are no HIV vaccines approved by the U.S. FDA.
Note: The article was updated on 2/2/24 to reflect accurate dates.
Our Trust Standards: Medical Advisory Committee