New Dengue Vaccine Gains WHO Recommendations
Dengue outbreaks continue to pose significant public health burdens in endemic countries and, unfortunately, may continue to increase in incidence as disease-carrying mosquitoes expand geographically, according to the World Health Organization (WHO).
To reduce the severity of dengue outbreaks, the WHO today announced the live-attenuated quadrivalent dengue vaccine Qdenga® (TAK-003) developed by Takeda has been confirmed to demonstrate efficacy against all four serotypes of the dengue virus in baseline seropositive children (4-16 years) in endemic countries.
And against serotypes 1 and 2 in baseline seronegative children.
The WHO's Strategic Advisory Group of Experts on Immunization (SAGE) on Immunization recommended on October 2, 2023, that Qdenga be considered for introduction in settings with high dengue disease burden and high transmission intensity to maximize the public health impact and minimize any potential risk in seronegative persons.
The SAGE now recommends introducing Qdenga to children aged 6 to 16.
The vaccine should be introduced within this age range about 1-2 years before the age-specific peak incidence of dengue-related hospitalizations.
Qdenga should be administered in a 2-dose schedule with a 3-month interval between doses.
The WHO will consider the SAGE recommendation and update its paper on dengue vaccines to include final guidance on using Qdenga in public vaccination programs.
"The global impact of dengue cannot be overlooked as the incidence continues to rise. This week, the WHO's SAGE provided important recommendations for the use of QDENGA in preventing dengue," commented Gary Dubin, M.D., president of the Global Vaccine Business Unit at Takeda, in a press release on October 3, 2023.
While approved for use in Brazil and various European countries, Qdenga is unavailable in the U.S.
On July 11, 2023, Takeda announced that the Company has voluntarily withdrawn Qdenga's U.S. Biologics License Application following discussions with the U.S. Food and Drug Administration on aspects of data collection.
However, the Dengvaxia® vaccine is both FDA and WHO-recommended where appropriate.
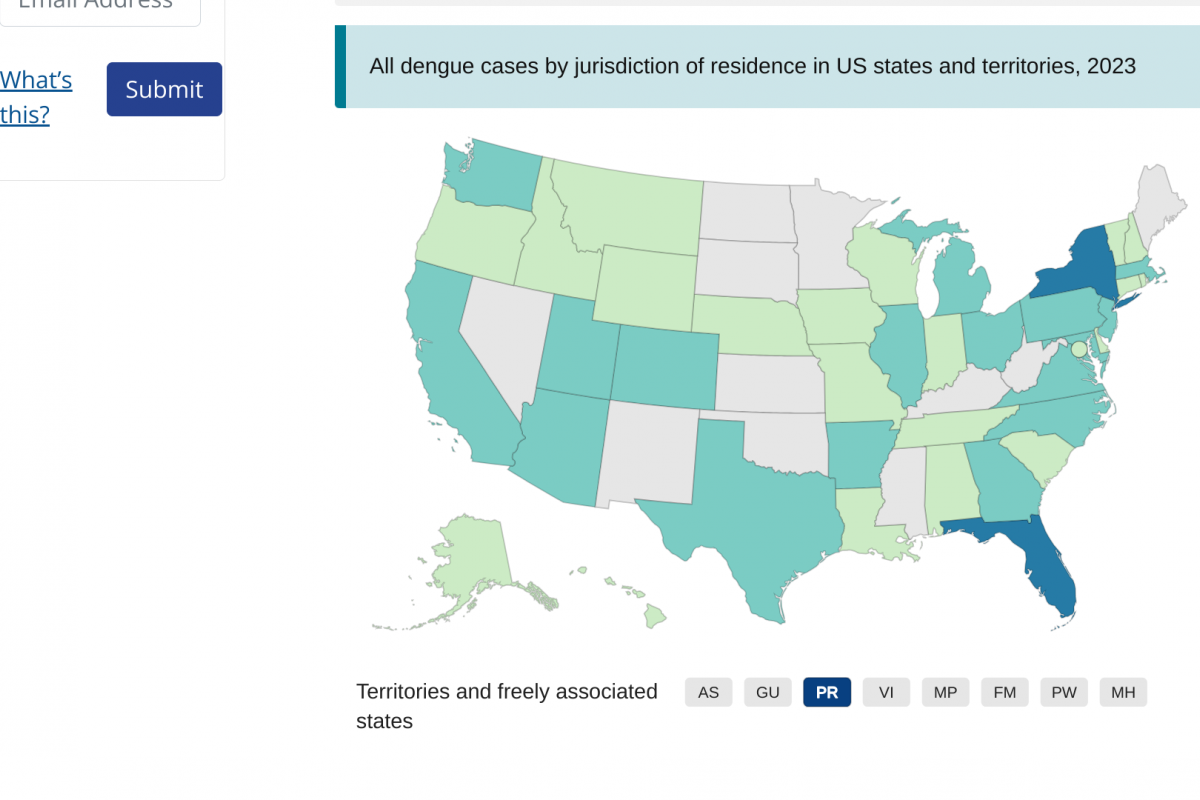
As of September 13, 2023, 44 U.S. jurisdictions had reported about 997 dengue cases this year. Throughout the summer of 2023, dengue outbreaks have been reported in southern Florida and Puerto Rica.
Note: This article was updated on October 3, 2023, to include the Company's press release.
Our Trust Standards: Medical Advisory Committee























