New Malaria Vaccine Gains Second Approval
The Nigerian National Agency for Food and Drug Administration and Control (NAFDAC) today announced its approval for the R21/Matrix-M™ Malaria Vaccine manufactured by India's Serum Institute of India Pvt. Ltd.
The Marketing Authorization Holder is Fidson Healthcare Ltd.
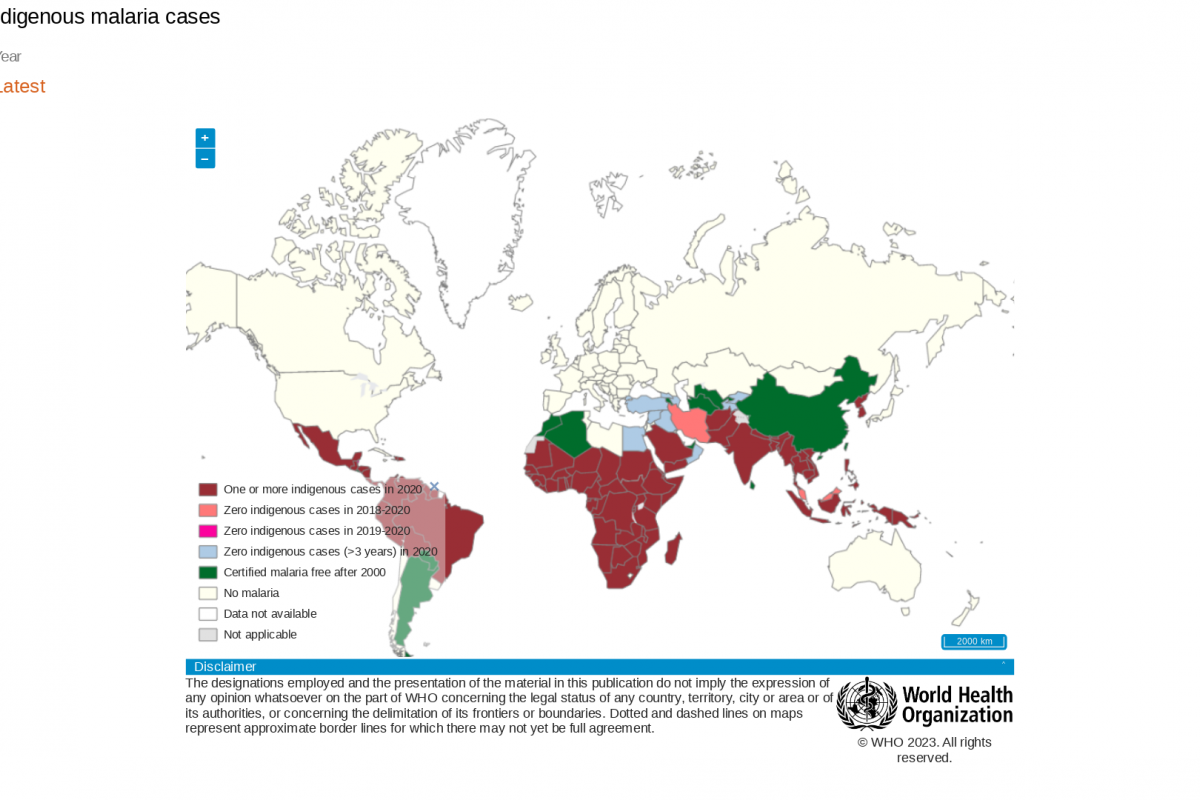
During a press briefing on April 17, 2023, Prof Mojisola Christianah Adeyeye, Director-General NAFDAC, said malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
According to the 2021 World Malaria Report, Nigeria had the highest number of global malaria cases (27%) and the highest number of related fatalities (32%) in 2020
This is the second authorization for R21/Matrix-M this month, following the Republic of Ghana.
GSK's Mosquirix™ RTS,S recombinant malaria vaccine was authorized in 2020.
Additional malaria vaccine and outbreak news are posted at Vax-Before-Travel.
Our Trust Standards: Medical Advisory Committee























