China Approves 2-Dose HPV Vaccinations For Young Women

London-based GSK plc announced a two-dose schedule for its Human Papillomavirus (HPV) vaccine Cervarix Vaccine was approved by China's National Medical Products Administration (NMPA) for young women aged 9 to 14.
With this approval announced on May 27, 2022, Cervarix is the first imported two-dose HPV vaccine for this age group in mainland China.
The NMPA authorization of the two-dose regimen adds China to two-dose approvals in approximately 100 countries, including the European Union, Asia, Africa, and Latin America.
The three-dose schedule for Cervarix [Human Papillomavirus bivalent (types 16, 18) Vaccine, Recombinant)], a non-infectious recombinant, AS04-adjuvanted vaccine, remains on the label for girls and women aged 15-45 years in China.
In 2020, there were approximately 110,000 new cases of cervical cancer and 59,000 deaths due to the disease in China.
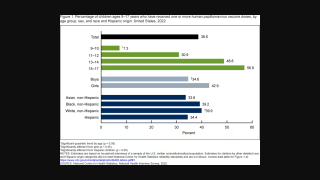
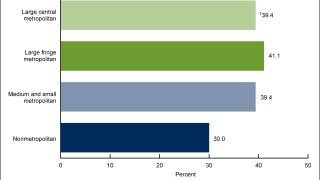
In the U.S., HPV vaccination for adolescents has been recommended for females since 2006 and males since 2011.
As of February 17, 2022, the updated HPV vaccine recommendations and dose schedules are found on this U.S. CDC webpage.
Additional HPV vaccine news is posted at PrecisionVaccinations.com/HPV.
Note: GSK's announcement was manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee