HIV Patients May Not Need 3rd Measles Vaccination

According to researchers from Johns Hopkins University, there is no difference in measles seroprevalence between HIV-infected and -uninfected patients.
These researchers wrote ‘Based on similar measles seroprevalence between HIV-infected and HIV-uninfected adolescents and adults, and the low response to vaccination, the studies reviewed do not support the need for an additional dose of measles-containing vaccine (MCV) in HIV-infected adolescents and adults.'
HIV stands for human immunodeficiency virus, which weakens a person’s immune system by destroying important cells that fight disease and infection.
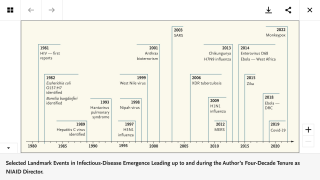
This is important news since there is a resurgence of measles cases around the world, such as in Israel and New York during 2018.
This study is a systematic review of 30 studies meeting inclusion criteria to synthesize available evidence regarding measles seroprevalence and measles vaccine immunogenicity, efficacy, and safety in HIV-infected adolescents and adults, and was published in Clinical Infectious Disease on November 17, 2018.
This study’s conclusion differs from the World Health Organization (WHO) recommendation.
The WHO recommends an additional dose of MCV for HIV-infected children receiving highly active antiretroviral therapy (HAART) following immune reconstitution.
However, this new finding does support WHO guidelines that say ‘measles vaccine should be administered to potentially susceptible, asymptomatic HIV-infected adults, and may be considered for those with symptomatic HIV infection, if not severely immunosuppressed.'
Additionally, there are several infectious disease preventive vaccines recommended for HIV infected adults
But, there is currently no vaccine available that will prevent HIV infection or treat those who have it.
However, scientists are working to develop an HIV vaccine.
There are current HIV vaccine candidate clinical trials underway, such as HVTN702.
HVTN702 is a pivotal Phase 2b/3 ALVAC/Bivalent gp120/MF59, called HVTN 702, is testing whether an experimental vaccine regimen safely prevents HIV infection among adults.
“If deployed alongside our current armory of proven HIV prevention tools, a safe and effective vaccine could be the final nail in the coffin for HIV,” said Anthony S. Fauci, M.D., director of the NIAID, part of the NIH.
Our Trust Standards: Medical Advisory Committee