Innovative Pneumococcal Conjugate Vaccine for Adults Approaches Approval
Since its discovery by Louis Pasteur in the 1880s, S. pneumoniae has become the leading global cause of morbidity and mortality across all age groups, with seniors being particularly vulnerable.
Over the years, vaccines have prevented significant diseases, with innovative pneumococcal vaccines on the horizon.
At the 13th Meeting of the International Society of Pneumonia and Pneumococcal Diseases (ISPPD) in Cape Town, South Africa, Merck announced positive data from multiple Phase 3 studies evaluating V116, the company's investigational, adult-specific 21-valent pneumococcal conjugate vaccine.
Across the studies presented, V116 was shown to be immunogenic for all 21 serotypes covered by the vaccine in a variety of adult populations, such as those who had not previously received a pneumococcal vaccine (pneumococcal vaccine-naïve), those who had previously received a pneumococcal vaccine (pneumococcal vaccine-experienced) and those with an increased risk of pneumococcal disease, including people living with human immunodeficiency virus (HIV).
In all STRIDE studies presented at the meeting, V116 elicited higher immune responses than the studied comparators for the serotypes unique to V116.
Several of the studies presented at ISPPD were included in Merck's recent filing submission to the U.S. Food and Drug Administration (FDA).
The FDA granted V116 priority review with a Prescription Drug User Fee Act of June 17, 2024. V116 would be the first pneumococcal conjugate vaccine designed for adults if approved.
"The extensive data presented this week reaffirm our confidence in the potential clinical value V116 could provide to a range of adult populations," said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, in a press release on March 19, 2024.
"We are encouraged by the results of these studies showing that V116 has generated immune responses to the serotypes responsible for most adult invasive pneumococcal disease."
In addition to Phase 3 clinical data on V116, Merck also presented preliminary data from the real-world evidence study in the U.S., Pneumococcal Pneumonia Epidemiology, Urine Serotyping, and Mental Outcomes (PNEUMO), which found that among 2,065 adults 50 years of age and older hospitalized with community-acquired pneumonia between 2018 and 2022, 242 pneumococcal serotypes were detected.
Of these serotypes, approximately 84% were covered by V116.
Approximately 25% of the detected serotypes were covered only by V116, not PCV15 or PCV20.
Results from the PNEUMO study support that the serotypes in V116 account for most pneumococcal disease, including invasive and non-invasive, in adults 50 and older, says Merck.
An overview of the V116 late-stage development program is available at this Merck link.
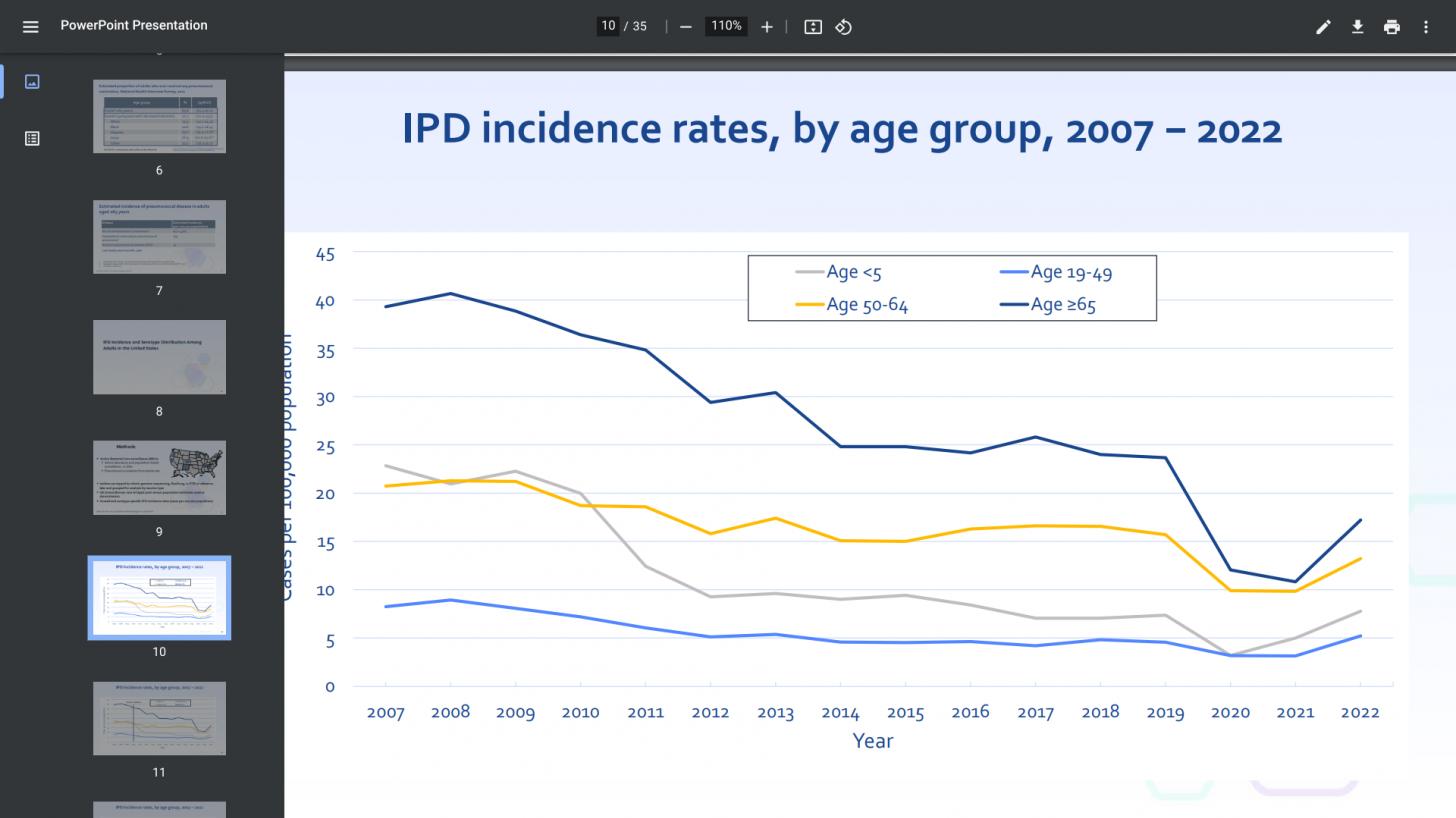
According to the U.S. CDC, pneumococcal disease is an infection caused by Streptococcus pneumoniae. There are more than 100 different serotypes of pneumococcal bacteria, which can affect adults differently than children.
While healthy adults can suffer from pneumococcal disease, patient populations particularly vulnerable to infection include older adults and those with certain chronic or immunocompromising health conditions, such as heart disease, lung disease, and liver disease. Mortality from invasive pneumococcal disease is highest among adults 50 years of age and older.
During the CDC's vaccine committee meeting on February 2024, Ryan Gierke, MPH, presented: Current Epidemiology of Pneumococcal Disease among Adults. The presentation's conclusion included PCV21 has greater coverage of the serotypes causing IPD in adults compared to PCV20, which covers 54–58% of adult IPD, while PCV21 covers 81–84% of adult IPD.
As of March 2024, the U.S. Food and Drug Administration, the European Medicines Agency, and the U.K.'s NHS have approved various vaccines to prevent pneumococcal disease.
Our Trust Standards: Medical Advisory Committee
























