Clinical Studies Support Doxy-PREP Use Against Chlamydia, Gonorrhea, and Syphilis

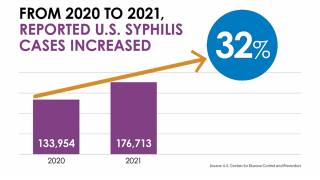
Several health reports have recently confirmed that sexually transmitted infections (STIs) have reached a record high for an eighth consecutive year.
Cases of chlamydia, gonorrhea, and syphilis have been impacting the health of teenagers, college students, and young adults more than ever.
The U.S. Centers for Disease Control and Prevention (CDC) reported these bacteria-related STIs reached more than 2.5 million people over the past year.
Since there are no approved vaccines available for these STIs, the CDC announced on October 2, 2023, that it was opening docket CDC-2023-0080 to obtain comments on proposed guidelines for the use of doxycycline post-exposure prophylaxis (PEP) for the prevention of bacterial STI.
Regarding preventive treatment options, doxycycline postexposure prophylaxis (doxy-PEP) has shown promising results.
The CDC's draft proposal recommends the antibiotic doxycycline as a "morning-after-pill" to help prevent chlamydia, syphilis, and gonorrhea infections among high-risk people.
Previously, the U.S. NIH announced in April 2023 that taking doxycycline within three days after unprotected sex reduced the risk of STIs among those at increased risk.
Furthermore, in August 2023, the CDC listed numerous clinical studies that have been conducted, offering insights into the benefits of doxy-PREP to prevent bacterial STIs. MEDLINE/PubMed and Embase were searched using the various terms.
The good news is that approved vaccines are available in December 2023 for certain sexually transmitted diseases, such as hepatitis, human papillomavirus, and mpox. These vaccines are available at most clinics and pharmacies in the U.S.
Vaccine candidates targeting Epstein-Barr Virus, Herpes, and HIV are continuing clinical research in 2023.
Our Trust Standards: Medical Advisory Committee