Accurately Diagnosing Typhoid Requires Enhanced Testing

A new study focused on typhoid and paratyphoid fevers indicates cases can be missed when patients don't seek medical care, or seek medical care and don't have a blood culture test.
Which means, the true burden of typhoid related disease could be underestimated.
This is important news since the World Health Organization (WHO) estimates about 22 million cases of typhoid fever and 5 million cases of paratyphoid fever occur worldwide each year, causing about 200,000 fatalities.
Typhoid and paratyphoid fever are infections caused by the bacteria Salmonella enterica Typhi and S. Paratyphi.
Researchers writing in PLOS Neglected Tropical Diseases on January 16, 2020, have now calculated inflation factors that can be used to adjust these typhoid incidence rates to account for under-detection.
Merryn Voysey, the Lead Statistician at the Oxford Vaccine Group, University of Oxford, UK, and colleagues used data from an ongoing Typhoid Vaccine Acceleration Consortium (TyVAC) clinical trial of a typhoid vaccine in Nepal.
During the first year of passive surveillance, data was collected on 2,393 fever presentations. Overall, 1615 (68%) of patients had blood cultures.
The models used in this study revealed that patients who had blood taken were 1.87 times more likely to be positive for Salmonella than those without blood cultures.
"Crude typhoid incidence estimates should be adjusted for both the proportion of cases that go undetected due to missing blood cultures as well as the lower likelihood of culture-positivity in the group with missing data," the researchers say.
In the UK, the NHS says a diagnosis of typhoid fever can usually be confirmed by analyzing samples of blood, stools, or urine. These will be examined for the Salmonella typhi bacteria that cause the condition.
In countries with a high incidence rate of typhoid-related diseases, vaccine programs are used to control typhoid fever.
To address this need, the WHO issued a revised policy on typhoid vaccines in 2018. This policy follows evidence-based recommendations that prevention through vaccination is one of the most effective solutions to reduce the burden of typhoid in endemic areas.
In a previous, related study of the typhoid conjugate vaccine (TCV) December 5, 2019, showed a single vaccination to be 81 percent safe and effective in reducing typhoid in children between the ages of 9 months and less than 16 years.
The researchers noted that these were preliminary results and that the study will continue to follow the participants for the next 2 years.
The US Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices recommends 2 typhoid fever vaccines, an oral vaccine, Vivotif, and an injectable vaccine, Typhim VI.
Neither vaccine is 100 percent effective, so travelers to endemic countries should also practice safe eating and drinking habits while traveling abroad, says the CDC.
Travelers to Asia, Africa, and Latin America are especially at risk, and the highest risk for typhoid is in south Asia.
If you get sick after returning to the United States, seek medical care and tell your healthcare provider where and when you traveled, says the CDC.
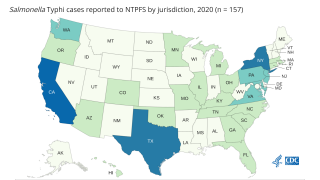
In the USA, approximately 350 culture-confirmed cases of typhoid fever and 90 cases of paratyphoid fever caused by Paratyphi A are confirmed annually.
Study funding: The authors thank the Bill & Melinda Gates Foundation (OPP1151153) for funding TyVAC, including the Nepal trial; the Wellcome Trust and the Bill & Melinda Gates Foundation for funding of The Strategic Typhoid Alliance across Africa and Asia, which supported the surveillance that underpins this trial; and the National Institute for Health Research Oxford Biomedical Research Centre. MV is funded by a National Institute of Health Research (NIHR) Doctoral Research Fellowship (DRF-2015-08-048). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
The researchers disclosed various industry relationships.
The Typhoid Vaccine Acceleration Consortium (TyVAC), a partnership between the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine, the Oxford Vaccine Group at the University of Oxford, and PATH, an international nonprofit, aims to accelerate the introduction of new typhoid conjugate vaccines (TCVs) as part of an integrated approach to reducing the burden of morbidity and mortality from typhoid in countries eligible for support from Gavi, the Vaccine Alliance.
Typhoid travel vaccine news published by Vax-Before-Travel
Our Trust Standards: Medical Advisory Committee