11 Tuberculosis Vaccine Candidates Nearing Completion

As World Tuberculosis Day approaches on March 24, everyone should focus on preventing this disease to reduce its impact on society.
According to GlobalData, an analytics company, various TB vaccines are in late-stage development and hold promise in containing the disease burden.
GlobalData reported today that there are currently 11 TB vaccine candidates in the late stages of development.
For example, the M72/AS01E vaccine candidate could be the first licensed TB vaccine in decades.
While the 100-year-old Bacillus Calmette-Guérin (BCG) vaccine is up to 80% effective at preventing TB infection in young children, it provides reduced protection against pulmonary TB.
This means there is a significant need for improved prophylactic vaccines.
Currently, there are about 16 approved TB vaccines in use worldwide.
Anaelle Tannen, Infectious Disease Analyst at GlobalData, commented in a press release on March 22, 2024, "Progress in this area has the potential to save countless lives as well as reduce the health and socio-economic burden associated with this disease."
Tannen added, "Prevention and early diagnosis are key to stopping the ongoing spread of the disease. The BCG vaccine is currently the only prophylactic on the market. It is given to babies in countries where TB is common."
"In areas where TB is less common, it is only given to those at high risk, including those that are more likely to be exposed to the bacterium."
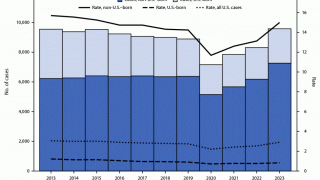
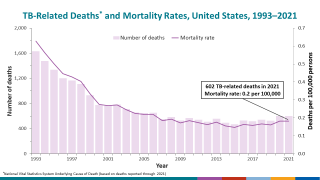
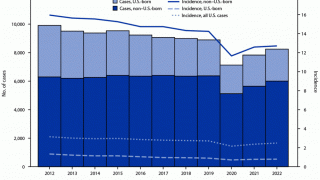
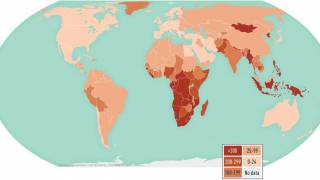
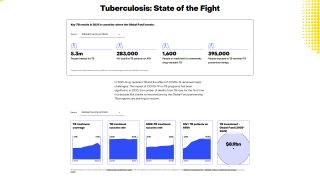
TB remains a global pandemic, with 1.8 billion people estimated to be infected with the bacteria, according to the World Health Organization (WHO). India leads most countries in reporting TB cases and deaths.
The disease exists in both a latent and active form; the latent type does not express symptoms and is not transmissible unless it develops into the active type.
The lifetime risk for latently infected persons is about 5-10%.
Antibiotics are typically administered for at least six months, and ensuring the course is completed is vital to prevent antibiotic resistance, says the WHO.
In the United States, the TICE® BCG vaccine is available at most health departments but not retail pharmacies.
Our Trust Standards: Medical Advisory Committee