New Multi-Valent HPV Vaccine Research Launches

Merck today announced plans to initiate clinical development of a new investigational multi-valent human papillomavirus (HPV) vaccine designed to provide broader protection against multiple HPV types.
Separately, the company also plans to conduct clinical trials to evaluate the efficacy and safety of a single-dose regimen of GARDASIL®9 compared to the approved three-dose regimen.
“Evidence continues to emerge showing the importance of GARDASIL and GARDASIL 9 to public health,” said Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, in a press release on March 13, 2024.
“These significant investments build upon our leadership and, importantly, provide the opportunity to further impact the global burden of certain HPV-related cancers and diseases.”
Merck announced in February 2024 that GARDASIL/GARDASIL 9 vaccine sales reached about $8.9 Billion;
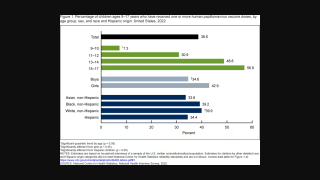
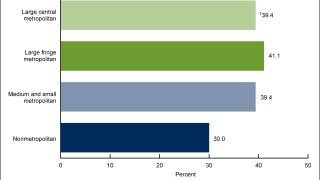
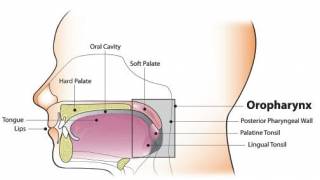
The World Health Organization (WHO) recommends vaccinating against HPV to prevent infections and associated cancers.
As of March 2024, the WHO has listed six licensed HPV vaccines protect males and females against cancers caused by HPV. These bivalent, quadrivalent, and nonavalent HPV vaccines are available in 140 countries.
Furthermore, the WHO and the United Kingdom have endorsed the single-dose regimen.
Our Trust Standards: Medical Advisory Committee