Sudan Ebolavirus Vaccine Candidate Testing Begins in the U.S.

IAVI announced today that the initial participants had been vaccinated with a Sudan virus (SUDV) vaccine candidate in a first-in-human Phase I clinical trial in the U.S.
As of June 27, 2023, the IAVI C108 IAVI-sponsored trial is funded by the Biomedical Advanced Research and Development Authority (BARDA).
IAVI C108 will occur at two U.S.-based clinical trial sites, where the vaccine candidate will be administered intramuscularly at three dosage levels.
This is essential news since there are no SUDV vaccines available.
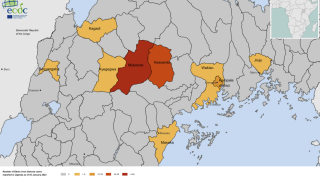
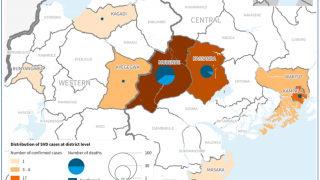
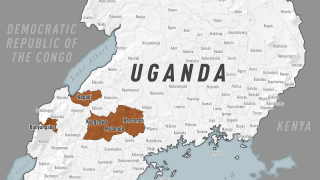
Furthermore, like the Zaire Ebolavirus (ZEBOV), SUDV is responsible for recurring viral hemorrhagic fever outbreaks across sub-Saharan Africa.
In past Ebola outbreaks, the estimated case fatality ratios of SUDV disease have varied from 41% to 100%.
This study evaluates the safety and immunogenicity of an investigational SUDV vaccine candidate previously donated to IAVI by Merck. This investigational SUDV vaccine candidate was produced for IAVI from an existing investigational bulk drug substance previously manufactured by Merck.
IAVI is responsible for all aspects of the candidate’s future development, including demonstrating equivalence between this SUDV vaccine candidate and IAVI’s other SUDV vaccine candidate, which utilizes the same viral vector but is manufactured using a new production platform.
The SUDV vaccine candidate being evaluated in IAVI C108 uses the same recombinant vesicular stomatitis virus (rVSV) viral vector platform as ERVEBO®, Merck’s single-dose ZEBOV vaccine, which is licensed in the U.S., U.K., European Union, Canada, Switzerland, and 10 African countries.
“IAVI C108 represents an important first step toward generating the data needed for eventual licensure of an rVSV-SUDV vaccine. The development and licensure of ERVEBO® have resulted in an important tool in Ebola Zaire outbreak responses. If proven effective, we’re hopeful that a vaccine candidate built on the same viral platform will be similarly important in future SUDV outbreaks,” said Swati Gupta, Ph.D., vice president and head of emerging infectious diseases and epidemiology at IAVI, in a related press release.
The rVSV platform has been used extensively in adults and children. The underlying vesicular stomatitis virus is a common animal virus that does not cause serious illness in humans and has been investigated extensively as a vaccine vector.
In the vaccine platform, it is engineered to encode a surface protein from a target pathogen, in this case, SUDV, to prompt the body to mount an immune response.
Much of the research and development on IAVI’s rVSV platform is performed at the IAVI Vaccine Design and Development Lab in Brooklyn, New York.
Our Trust Standards: Medical Advisory Committee