$110 Million Awarded for Sudan Ebolavirus Therapeutic Development

The U.S. Administration for Strategic Preparedness and Response (ASPR) recently announced a $109.8 million contract with California-based Mapp Biopharmaceutical Inc. for the advanced development and potential purchase of the MBP134 monoclonal antibody therapeutic to treat Sudan ebolavirus (SUDV).
MBP134 is the first combination of monoclonal antibodies (mAbs) in BARDA's portfolio that could treat SUDV infections.
In patients diagnosed with SUDV, the mAbs bind to proteins on the viral surface and prevent the spread of infection.
"One of the ways we enhance the nation's readiness for health emergencies is by investing in medical countermeasures for which there is no commercial market," said ASPR Dawn O'Connell in a press release on October 4, 2022.
"If approved, this treatment will put the U.S. in a better position to prepare for and respond to future potential ebolavirus incidents."
"Given the current outbreak of Ebola Sudan in Uganda, this work is even more important."
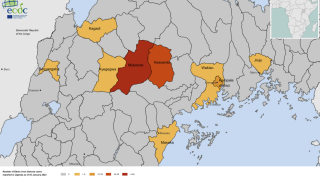
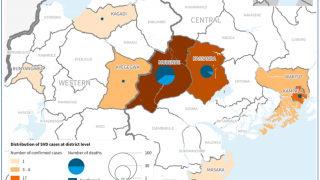
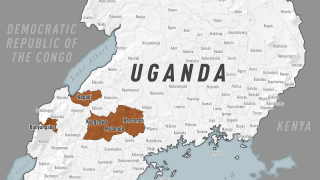
On September 20, 2022, Uganda health authorities declared an outbreak following confirmation of a SUDV case in Mubende.
Since then, about twenty-nine people, including four health workers, have died from an infection.
Given the four reported SUDV outbreaks since 2000, including the current outbreak in Uganda, developing therapeutics that address SUDV infections is critical to U.S. public health preparedness.
SUDV is an Ebola virus, a member of the filovirus family, and is part of a wider group of diseases known collectively as viral hemorrhagic fevers.
In 2006, the Department of Homeland Security determined ebolavirus to be a material threat to national security.
As of October 7, 2022, there are no approved products to treat SUDV infections.
Other Ebola vaccines and therapeutics supported by the U.S. government to treat Zaire ebolavirus have not been studied or approved for the prevention or treatment of SUDV disease.
The new ASPR funding under this award supports activities necessary to request regulatory approval of MBP134, including support for manufacturing, efficacy, and safety evaluations and regulatory submissions.
Under the contract, the company will develop a lyophilized (freeze-dried) formulation of MBP134 to help reduce the need for cold-chain storage and make transporting the therapeutic easier worldwide.
If the product receives U.S. Food and Drug Administration approval as a treatment for Sudan virus infection, BARDA will purchase the product for use in the United States as needed.
Additional Ebola vaccine and mAbs news are posted at Vax-Before-Travel.com/Ebola.
Note: This ASPR announcement was manually curated for mobile readers and is not paid content.
Our Trust Standards: Medical Advisory Committee