Updated Recommendations for the Prevention and Control of Influenza in Children

The American Academy of Pediatrics (AAP) announced recommendations for the routine use of influenza vaccine and antiviral medications to prevent and treat influenza in children during the 2021–2022 influenza season.
The AAP stated on September 7, 2021, it recommends annual influenza immunization of all children without medical contraindications, starting at six months of age.
Excerpts from the AAP statement are discussed below:
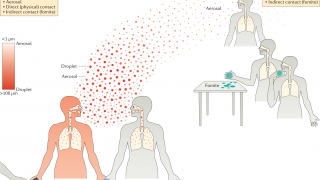
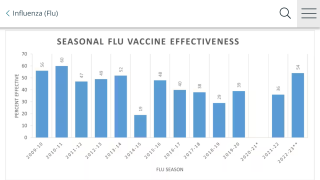
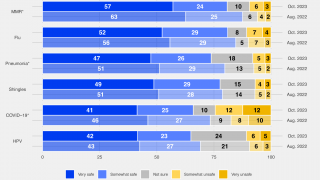
Influenza vaccination is necessary to protect vulnerable populations and reduce the burden of respiratory illnesses during the circulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is expected to continue during the 2021–2022 flu season.
According to the AAP, any licensed recommended age-appropriate influenza vaccine available can be administered without preference for one product or formulation over another.
Furthermore, antiviral treatment of influenza with any licensed, recommended, age-appropriate influenza antiviral medication is recommended for children with suspected or confirmed influenza who are hospitalized, have severe or progressive disease, or have underlying conditions that increase their risk of complications of influenza.
And, antiviral treatment may be considered for any previously healthy, symptomatic outpatient, not at high risk for influenza complications, in whom an influenza diagnosis is confirmed or suspected if treatment can be initiated within 48 hours of illness onset, and for children whose siblings or household contacts either are younger than six months or have a high-risk condition that predisposes them to complications of influenza.
Moreover, influenza vaccines may be administered simultaneously with or any time before or after administration of the current U.S. FDA Approved or Authorized COVID-19 vaccines.
Given that it is unknown whether reactogenicity of COVID-19 vaccines will be increased with coadministration of influenza vaccine, the reactogenicity profile of the vaccines should be considered, and providers should consult the most current CDC Advisory Committee on Immunization Practices (ACIP)/AAP guidance regarding coadministration of COVID-19 vaccines with influenza vaccines.
'No data are currently available concerning coadministration of currently Authorized COVID-19 vaccines and influenza vaccines,' stated Lisa Grohskopf, M.D., MPH, ACIP vaccine committee presentation on June 24, 2021.
However, children with acute moderate or severe COVID-19 should not receive the influenza vaccine until they have recovered. But children with mild illness may be vaccinated, says the AAP.
This APP policy statement summarizes updates and recommendations for the 2021–2022 influenza season. In addition, a detailed review of the evidence supporting these recommendations is published in the technical report.
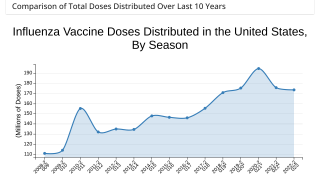
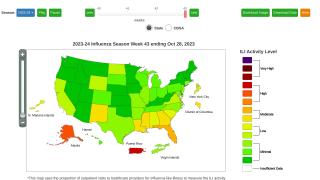
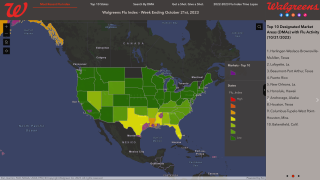
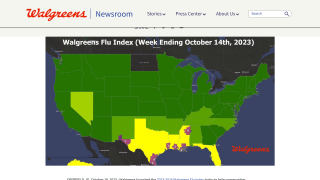
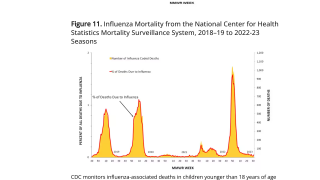
Note: As of September 10, 2021, the U.S. CDC’s U.S. Influenza Surveillance Report confirmed one influenza-associated pediatric death occurring during the 2020-2021 season had been reported to CDC. This data contrasted with the 2019-2020 flu season when 199 influenza-associated pediatric deaths were confirmed.
PrecisionVaccinations publishes fact-checked research-based vaccine news.
Our Trust Standards: Medical Advisory Committee