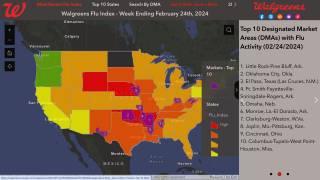
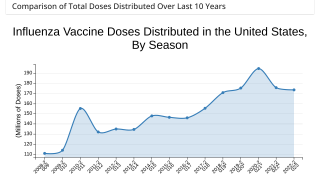
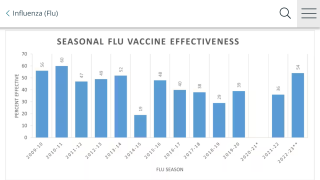
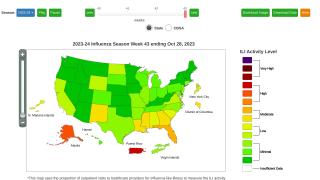
Cell-Based Flu Vaccines Offer the Best Match to Circulating Influenza Strains

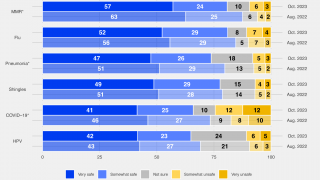
A recent study showed that higher levels of mismatch have consistently occurred with egg-derived influenza vaccine viruses when compared with cell-derived vaccine viruses over multiple influenza seasons.
This study presented by Seqirus on October 6, 2018, used publicly available reports from the Worldwide Influenza Centre, and evaluated the degree of match (antigenic similarity) between the circulating (wild-type) H3N2 influenza virus and reference (candidate vaccine virus) H3N2 virus derived from both eggs and mammalian cells over 12 influenza seasons.
The study results found that there was a higher proportion of similarity between the circulating influenza virus and the cell-derived reference virus compared to the egg-derived reference virus.
*Click here for an Asthma Clinical Trial*
Furthermore, for more than half of the influenza seasons evaluated, there was little or no antigenic similarity between the circulating H3N2 virus and the egg-derived H3N2 virus used to produce egg-based influenza vaccines.
“One of the challenges in fighting flu is that the viruses can change their genetic make-up rapidly -- not only between flu seasons but also during the course of a single season,” said FDA Commissioner Scott Gottlieb, M.D., in a separate statement during September 2018.
“As a result, the seasonal influenza vaccine needs to be evaluated annually to see whether its composition needs to be adjusted.”
“The release of these data follows the 2019 Southern Hemisphere strain selection meeting last week in Atlanta where the World Health Organization (WHO) for the first time recommended a change to the egg-derived H3N2 strain, but not to the cell-derived H3N2 strain,” said Ethan Settembre, Vice President of Research, Seqirus.
“The WHO decision is unprecedented and consistent with the data Seqirus presented at IDWeek during October 2018,” said Settembre in a press release.
According to the Centers for Disease Control and Prevention (CDC), growing influenza viruses in eggs can introduce changes that cause the body’s immune system to produce antibodies that are less effective at preventing diseases caused by circulating influenza viruses.
By maintaining the virus in cells from the initial isolation, cell-based influenza vaccines help avoid egg-adapted changes and may result in vaccines containing a virus that is more “like” wild-type circulating viruses.
Cell culture technology is potentially more flexible than the traditional technology, which relies upon an adequate supply of eggs, says the CDC.
According to the CDC, for the 2018-2019 flu season, providers may choose to administer any licensed, age-appropriate flu vaccine.
The vaccine options this season include:
- Standard dose flu shots. These are given into the muscle. They are usually given with a needle, but two (Afluria and Afluria Quadrivalent) can be given to some people (those aged 18 through 64 years) with a jet injector.
- High-dose shots for older people.
- Shots made with adjuvant for older people.
- Shots made with virus grown in cell culture.
- Shots made using a vaccine production technology (recombinant vaccine) that does not require the use of the flu virus.
- Live attenuated influenza vaccine (LAIV) – or the nasal spray vaccine – is also an option for use during the 2018-2019 season for persons whom it is otherwise appropriate.
Most pharmacies in the USA offer several FDA approved flu and vaccines. For the 2018-2019 flu season, the FDA says that providers may choose to administer any licensed, age-appropriate flu vaccine (IIV, RIV4, or LAIV4).
The CDC Vaccine Price List provides the private sector prices for general information.
Flu vaccine discounts can be found here.
Recent influenza vaccine news:
- Xofluza Beats Tamiflu in Phase 3 Clinical Study
- Cell-Culture and Egg-Based Flu Vaccines Similarly Un-Effective Against Influenza A
- High-Dose Flu Shot 30% More Effective at Reducing Senior Hospitalizations
Vaccines, like any medicine, can have side effects. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
For more information visit www.seqirus.com
Our Trust Standards: Medical Advisory Committee
- Statement by FDA Commissioner Scott Gottlieb, M.D., on preparations for the upcoming flu season and vaccinations
- Seqirus Presents Data at IDWeek Demonstrating Cell-Derived Viruses Have a Closer Match to Circulating Influenza Viruses Compared
- Recommended composition of influenza virus vaccines for use in the 2019 southern hemisphere influenza season
- Frequently Asked Flu Questions 2018-2019 Influenza Season
- How the Flu Virus Can Change: “Drift” and “Shift”
- Flu Vaccine Changes for the 2019 Southern Hemisphere