Multi-disease Vaccine Battling Mosquito Saliva

The National Institute of Allergy and Infectious Diseases (NIAID), has launched a clinical trial to test an investigational vaccine intended to provide broad protection against mosquito-transmitted diseases, such as Zika, malaria, West Nile fever and dengue fever.
Rather than targeting just one mosquito-borne disease, this vaccine candidate aims to protect people from the insect's bites.
"Mosquitoes cause more human disease and death than any other animal," said NIAID Director Anthony S. Fauci, MD.
"A single vaccine capable of protecting against the scourge of mosquito-borne diseases is a novel concept that, if proven successful, would be a monumental public health advance," said Dr. Fauci.
The investigational vaccine, called AGS-v, was developed by the pharmaceutical company SEEK, which has since formed a joint venture with hVIVO in London.
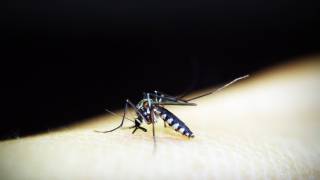
Unlike other vaccines targeting specific mosquito-borne diseases, the AGS-v candidate is designed to trigger an immune response to mosquito saliva rather than to a specific virus or parasite carried by mosquitoes.
The test vaccine contains four synthetic proteins from mosquito salivary glands. The proteins are designed to induce antibodies in a vaccinated individual and to cause a modified allergic response that can prevent infection when a person is bitten by a disease-carrying mosquito.
Led by Matthew J. Memoli, M.D., director of the Clinical Studies Unit in NIAID’s Laboratory of Infectious Diseases, the clinical trial is expected to examine the blood samples to measure levels of antibodies triggered by vaccination.
The mosquitoes will not be carrying viruses or parasites, so the participants are not at risk of becoming infected with a mosquito-borne disease. The mosquitoes will bite the participants’ arms through the netting on the feeding devices.
This study is expected to be completed by summer 2018. For more information about the trial, see ClinicalTrials.gov using the trial identifier NCT03055000.
Our Trust Standards: Medical Advisory Committee