Second Generation Dengue Vaccine Prequalified by the WHO

An innovative, second-generation vaccine for dengue prevention recently received prequalification from the World Health Organization (WHO).
Developed by Takeda GmbH, QDENGA® (TAK-003) (Dengue Tetravalent Vaccine [Live, Attenuated]) is an approved two-dose vaccine containing weakened versions of the four dengue serotypes that cause disease in people.
As of May 2024, QDENGA was launched in 21 countries and is available in 17 European countries but not the United States.
“The prequalification of TAK-003 is an important step in the expansion of global access to dengue vaccines, as it is now eligible for procurement by UN agencies, including UNICEF and PAHO,” said Dr. Rogerio Gaspar, WHO Director for Regulation and Prequalification, in a press release on May 15, 2024.
The WHO prequalification list also includes the Dengvaxia (CYD-TDV) vaccine developed by Sanofi Pasteur.
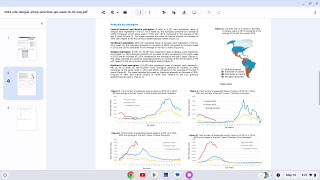
According to the WHO, the most significant number of dengue outbreaks reported was in 2023 with the WHO Region of the Americas reporting about 4.5 million cases and 2,300 deaths.
Our Trust Standards: Medical Advisory Committee