World’s Only Chikungunya Vaccine Ready To Address Significant Unmet Medical Need
Valneva SE today reported its consolidated financial results for the year ending December 31, 2023, and provided several key corporate updates. Valneva's commercial portfolio is composed of three travel vaccines, IXIARO®/JESPECT®, DUKORAL®, and IXCHIQ®.
Valneva raised its 2024 product sales guidance to between €160 million and €180 million due to an improved outlook regarding the IXIARO® supply constraints anticipated in February 2024.
The Company made excellent progress across the R&D pipeline with the U.S. FDA's approval of the single-shot chikungunya vaccine IXCHIQ®, the world's first and only vaccine to address this significant unmet medical need.
In today's press release, Peter Bühler, Valneva's Chief Financial Officer, commented, "In 2023, Valneva successfully executed key strategic objectives despite a difficult economic environment."
"Our chikungunya vaccine IXCHIQ® became the world's first licensed chikungunya vaccine available to address this significant unmet medical need, and we also managed to surpass our pre-pandemic product sales."
Valneva will host a live webcast of its full-year 2023 results conference call on March 20, 2024, at 3 p.m. CET/10 a.m. EDT. The webcast will also be available on the Company's website. Please refer to this link: https://edge.media-server.com/mmc/p/hom3riyt.
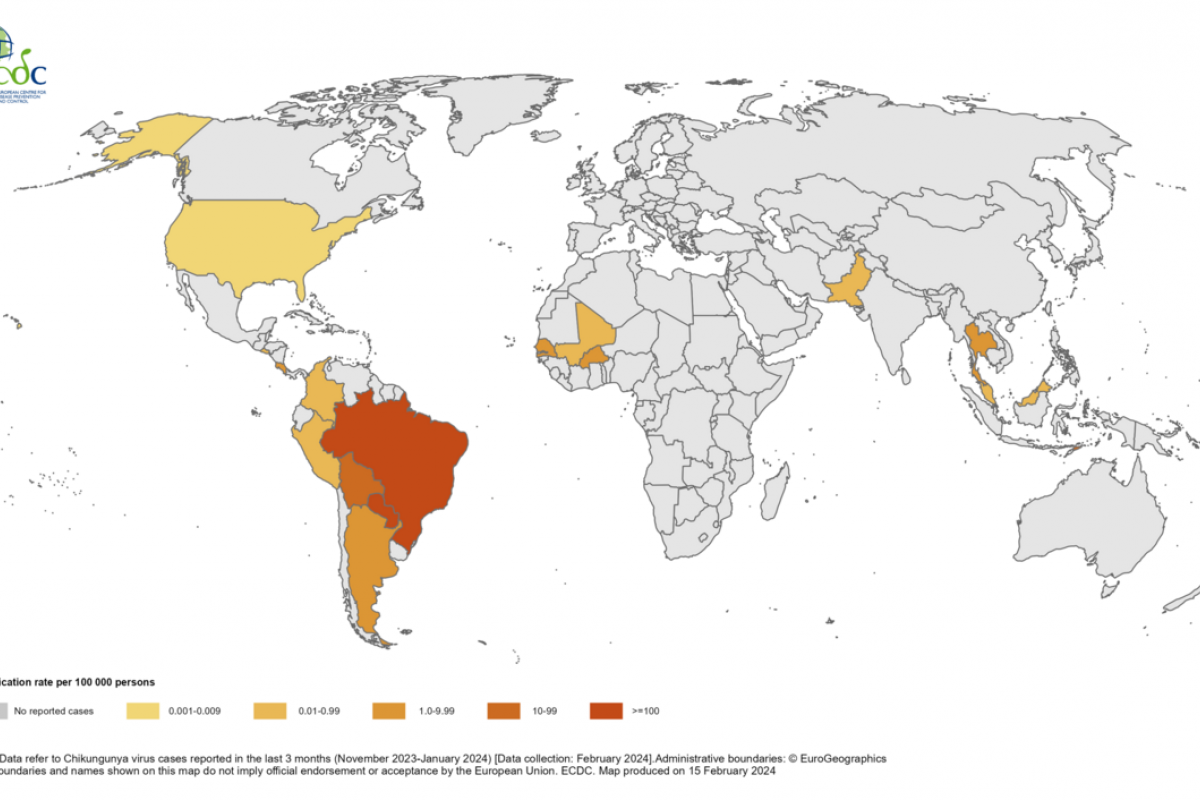
Chikungunya is a viral disease transmitted by mosquitoes infected with the chikungunya virus. The World Health Organization says chikungunya outbreaks were identified in nearly 115 countries, primarily in the Region of the Americas, in 2023.
Our Trust Standards: Medical Advisory Committee























