5-in-1 Meningococcal ABCWY Vaccine Candidate Could Simplify Immunizations

GSK plc recently announced positive results from a phase 3 clinical trial evaluating its MenABCWY combination vaccine candidate, administered as two doses given six months apart in healthy individuals aged 10-25 years.
GSK's MenABCWY vaccine candidate combines the antigenic components of its licensed meningococcal vaccines, Bexsero (MenB) and Menveo (MenACWY).
All primary endpoints were met, including the non-inferiority of the vaccine candidate for all five Neisseria meningitides serogroups (A, B, C, W, and Y) compared to licensed meningococcal vaccines Bexsero and Menveo in terms of an immune response.
In addition, the vaccine candidate was well tolerated, with a safety profile consistent with Bexsero and Menveo.
Tony Wood, Chief Scientific Officer at GSK, commented in a press release on march 14, 2023, "These statistically significant phase III data are a very encouraging step toward reducing the incidence of meningococcal disease."
"In the U.S., routine use of a 5-in-1 meningococcal vaccine with a two-dose regimen in adolescents at 16 to 18 years of age, just before this disease's incidence peak, could drive significant public health impact."
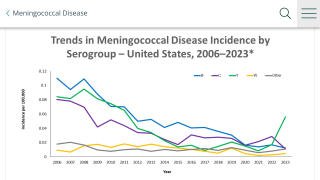
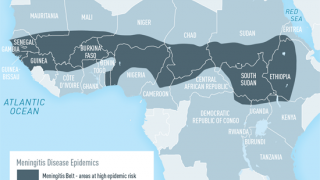
Invasive meningococcal disease (IMD), a significant cause of meningitis and septicemia, is an uncommon but serious illness that can cause life-threatening complications or even death, typically amongst previously healthy children and adolescents.
Five Neisseria meningitides serogroups (A, B, C, W, and Y) account for nearly all IMD cases worldwide.
Among those contracting meningococcal diseases, one in ten will die, sometimes in as little as 24 hours, despite treatment.
As yet, no licensed combination vaccine offers protection against these serogroups in a single vaccine.
Currently, in the U.S., two separate vaccines needing four injections are required to protect against all five serogroups.
This immunization regimen and low awareness of the disease can lead to sub-optimal immunization coverage rates, particularly for MenB, with an estimated coverage of only about 31% of adolescents in the U.S.
GSK works closely with regulators to review the complete phase III data set, including the supplemental Biologics License Application for Bexsero.
This clinical trial was the confirmatory trial for Bexsero and the phase III trial for MenABCWY.
Detailed results from this phase III trial will be presented in a peer-reviewed publication and at upcoming scientific meetings.
Our Trust Standards: Medical Advisory Committee