Invasive Meningococcal Disease Vaccine Approved

The U.S. Food and Drug Administration (FDA) announced its approval of a Biologics License Application for MenQuadfi Meningococcal (Groups A, C, Y, W) Conjugate Vaccine for the prevention of invasive meningococcal disease in persons 2 years of age and older.
This licensure on April 24, 2020, marks MenQuadfi as the only U.S. FDA-approved quadrivalent meningococcal vaccine indicated for persons 2 through 56 years of age and older.
And, MenQuadfi is the only quadrivalent meningococcal vaccine in the U.S. that uses tetanus toxoid as a protein carrier.
However, MenQuadfi does not prevent serogroup B disease.
David Loew, Executive Vice President, Sanofi Pasteur, said in a related press release, “Approval of this new vaccine in the U.S. represents an important milestone in the ongoing fight to help protect as many people as possible from meningococcal disease.”
“Meningococcal meningitis remains a major global health challenge because it can strike quickly and with devastating effect, taking a life in less than 24 hours.”
The ongoing Phase 3 trials are investigating use in infants as young as 6 weeks of age to better address the worldwide needs for meningococcal disease prevention throughout life.
The FDA approval is based on clinical data from 5 double-blind, randomized, multicenter Phase 2 and 3 trials that assessed the safety and immune responses following vaccination, with nearly 5,000 persons 2 years of age and older.
Based on study objectives, immune responses elicited by MenQuadfi achieved non-inferiority compared to those induced by licensed quadrivalent meningococcal vaccines.
Pivotal study results demonstrating MenQuadfi’s safety and effectiveness in inducing an immune response across all four serogroups have been published, including the performance of MenQuadfi in adolescents when the vaccine was co-administered with other routinely recommended vaccines, and its performance as a booster.
MenQuadfi is expected to be available to providers and pharmacies nationwide in the U.S. for immunization efforts in 2021.
In the U.S., the Centers for Disease Control and Prevention recommends vaccination against meningococcal disease at 11-12 years of age and a second dose at 16 years of age.
Despite strong public health recommendations, about half of teens have not received the recommended second dose of MenACWY vaccine by 17 years of age, leaving them vulnerable when they are at increased risk for the disease.
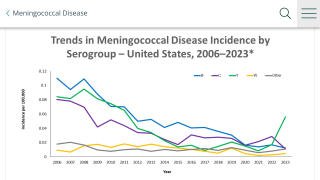
Hundreds of cases of vaccine-preventable meningococcal disease (caused by serogroups B, C, W, Y) still occur annually in the U.S. and, despite treatment, one in five survivors suffer from permanent complications such as hearing loss, organ damage, and limb amputations.
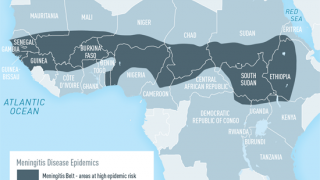
Around the world, meningococcal disease is highly unpredictable, and it varies widely across regions and ages.
MenQuadfi should not be given to people who have had a severe allergic reaction after a previous dose of MenQuadfi, any of its ingredients, or another vaccine that contains tetanus toxoid.
If MenQuadfi is given to people with a compromised immune system, including those receiving therapies that suppress the immune system, the immune response may be lower than expected.
Please see the full Prescribing Information.
Sanofi is dedicated to supporting people through their health challenges. We are a global biopharmaceutical company focused on human health.
Precision Vaccinations publishes breaking vaccine development news.
Our Trust Standards: Medical Advisory Committee