Why Delay Monkeypox Vaccination
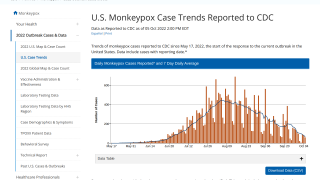
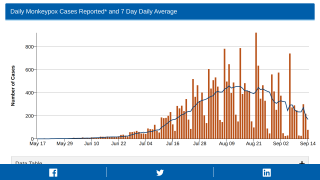
Since May 2022, monkeypox virus (MPV) infections have caught the global community's attention. However, this elevated concern is being tempered by a reducing number of confirmed MPV cases.
Over the past few weeks, fewer MPV cases have been reported daily.
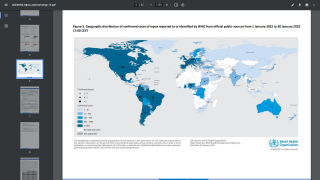
As of October 9, 2022, the U.S. CDC had reported 71,096 cases in over 100 countries but only 26 MPV-related fatalities.
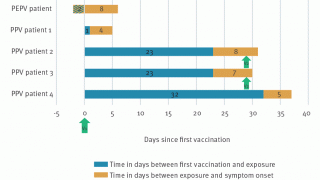
While the number of new infections is growing slowly ... waiting until the number of cases is high (again) would allow the MPV to adapt to humans, wrote researchers in a Correspondence published by the peer-review journal The Lancet.
Our analysis from first principles highlights the benefits of rapid intervention even for mild emerging pathogens..... just because a disease like monkeypox appears to be controllable does not mean it will stay controllable, added these researchers.
The initial constraints to monkeypox prevention vaccine access did generate ample frustrations between countries and cities.
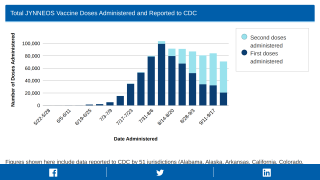
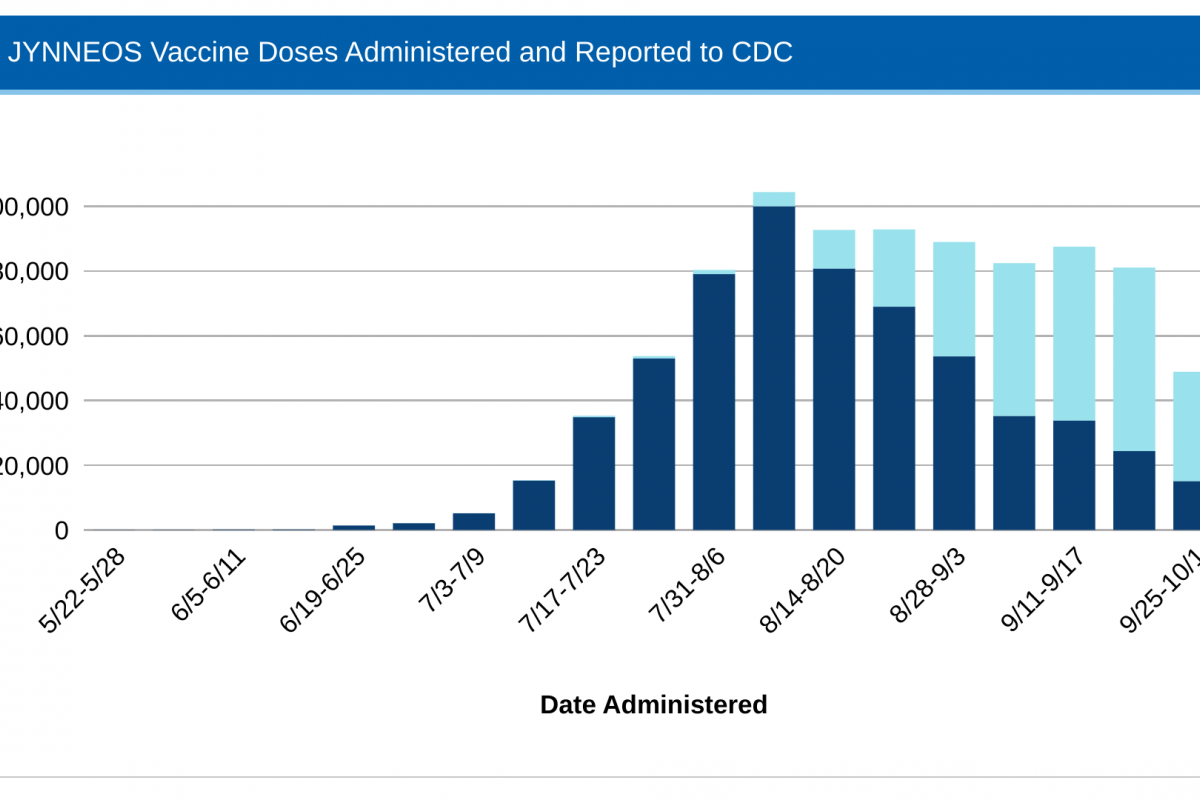
However, recent data from the CDC indicates MPV vaccine supply in the USA has caught up with demand.
The CDC recently confirmed that 873,552 doses of the U.S.-FDA-approved JYNNEOS vaccine had been administered in the 54 U.S. Jurisdictions reporting data.
This data closely matches the 838,513 JYNNEOS® (MVA-BN) doses distributed as of October 7, 2022.
Even in the hot-spot of New York City (NYC), access to monkeypox vaccines has improved.
On October 6, 2022, the New York City Department of Health and Mental Hygiene opened up 30,000 new vaccine appointments under new eligibility guidelines.
And NYC has adopted a preexposure prophylaxis model by expanding eligibility for MPV vaccination.
"As a community working closely together, we have radically reversed the trajectory of this virus," said Health Commissioner Dr. Ashwin Vasan in a recent press release.
"By introducing preexposure prophylaxis vaccination – or reaching people who may be exposed in the future – we will protect even more New Yorkers and continue to blunt this outbreak."
As of September 2022, the U.S. government had ordered 7 million vials of JYNNEOS from Bavarian Nordic to be delivered by mid-2023.
And to enhance main-street access, the U.S. HHS published a Public Readiness and Emergency Preparedness Act declaration granting pharmacists certain authorities to administer monkeypox vaccines.
JYNNEOS remains the only FDA-approved non-replicating monkeypox vaccine approved for military and non-military use.
As of September 2022, the CDC stated the peak immunity is expected to be reached 14 days after the second dose of JYNNEOS; however, the immunity duration is unknown.
In addition, no immune correlate of protection has been established by the CDC.
Other monkeypox vaccine news is posted at Monkeypox Today.
Note: The Lancet information was manually translated and curated for mobile readership.
Our Trust Standards: Medical Advisory Committee