USA Distributing 144,000 More Monkeypox Vaccines
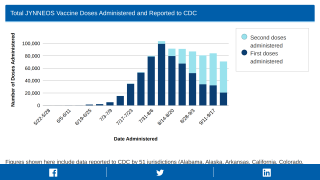
The U.S. Department of Health and Human Services (HHS) announced yesterday that it would distribute additional 144,000 doses of the monkeypox prevention Jynneos® vaccine to U.S. states and jurisdictions on July 11, 2022.
HHS has already delivered 41,000 Jynneos doses since June 28, 2022.
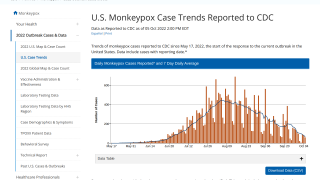
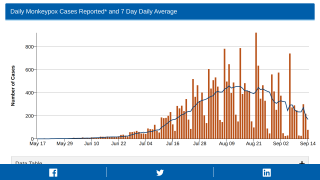
This new allocation is related to the U.S. CDC confirming that 700 people have been infected with the monkeypox virus (MPXV) as of July 7, 2022.
The leading states reporting MPXV outbreaks are California (136), New York (131), and Illinois (91).
The U.S. CDC says 'Monkeypox is a sylvatic zoonosis virus belonging to the Orthopoxvirus family.
"We are using every tool we have to increase and accelerate JYNNEOS vaccine availability in jurisdictions that need them the most," commented Steve Adams, Director of the Strategic National Stockpile, in a press release issued on July 7, 2022.
On July 1, 2022, the Biomedical Advanced Research and Development Agency ordered an additional 2.5 million doses of Jynneos to respond to current or future monkeypox outbreaks and as part of U.S. smallpox preparedness.
Altogether, HHS anticipates making approximately 1.9 million doses of Jynneos available in 2022, with an additional 2.2 million available during the first half of 2023.
Bavarian Nordic's Jynneos smallpox - Monkeypox vaccine is based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara.
The two-dose Jynneos (MVA-BN, IMVANEX®, Europe; IMVAMUNE®, Canada) was Approved by the U.S. FDA in 2019 and by the European Medicines Agency in 2013.
The CDC says Jynneos can be used for PEP, PEP++, or PrEP, following risk-benefit discussions and a review of any conditions that could increase the risk for serious adverse events.'
However, as of July 8, 2022, 'We do not know if Jynneos will fully protect against monkeypox virus infection.'
To better detect MPXV infections, more than 70 Laboratory Response Network labs in 48 states and five major commercial reference labs increased testing capacity with U.S. FDA-cleared orthopoxvirus tests.
The first commercial lab, Labcorp, announced on July 6, 2022, began offering orthopoxvirus testing in clinics and online.
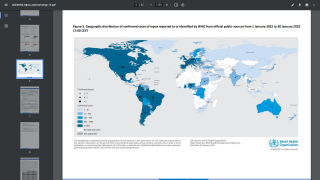
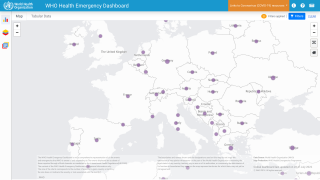
Internationally, data sources indicate over 8,200 MPVX patients have been confirmed since early May 2022.
Additional monkeypox outbreak news is posted at Vax-Before Travel.
Note: The HHS announcement was manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee