Chikungunya Virus Virus-like Particle Vaccine Candidate Approaches Finish LIne
Maryland-based Emergent BioSolutions Inc. announced on October 15, 2021, the first participant was dosed in its pivotal phase 3 study evaluating the safety and immunogenicity of the company's investigational chikungunya virus (CHIKV) virus-like particle (VLP) vaccine candidate, CHIKV VLP.
CHIKV VLP is the only single-dose, VLP-based vaccine currently in clinical development for active immunization against chikungunya disease.
The goal of this multi-center, randomized, double-blind, placebo-controlled study with 3,150 participants in up to 49 U.S. sites is to evaluate the safety and immunogenicity of the CHIKV VLP vaccine candidate in healthy individuals aged 12 to 64 as well as to demonstrate the consistency of the CHIKV serum neutralizing (SNA) antibody response across three manufactured vaccine candidate lots.
The study will observe the CHIKV SNA response at day 22, as measured by geometric mean titer and seroresponse rate.
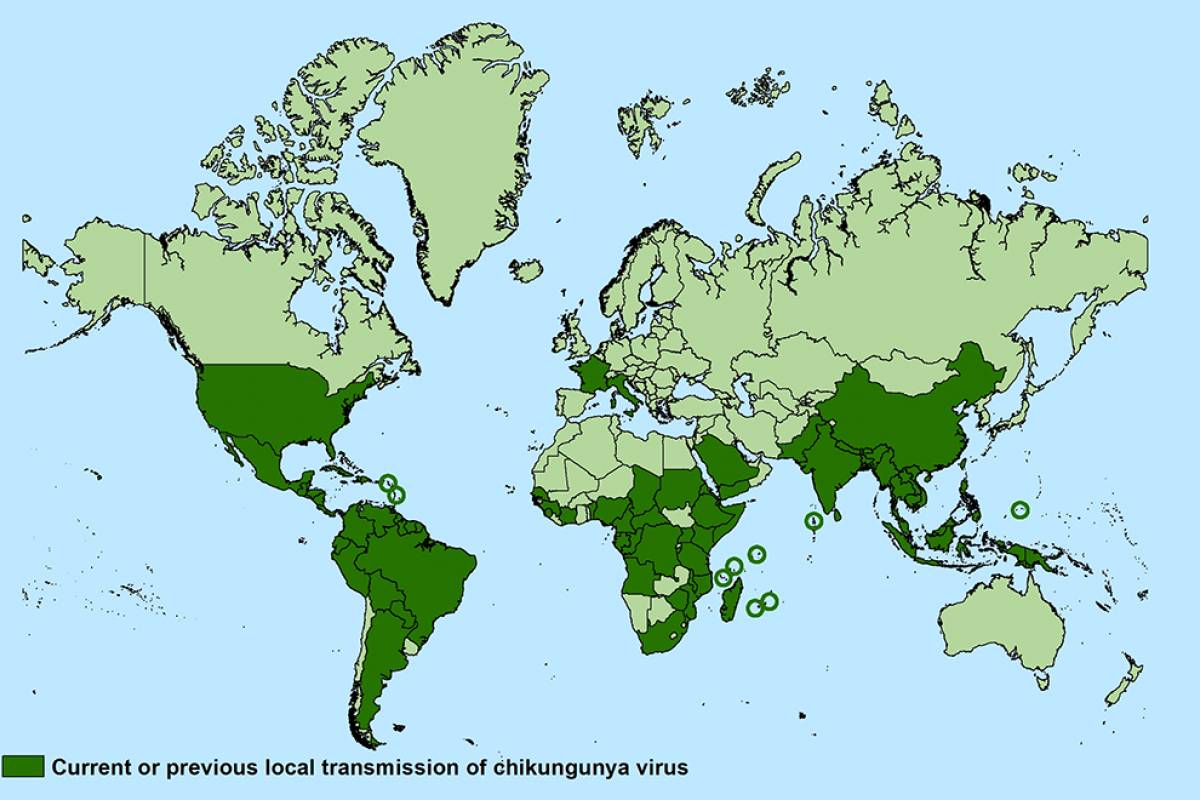
Chikungunya virus symptoms, which are spread by infected mosquitoes, include fever, joint pain, headache, muscle pain, joint swelling, or rash, with some symptoms lasting months and years, says the U.S. CDC.
The geographic distribution of CHIKV has expanded to more than 100 countries and territories worldwide.
As of October 19, 2021, the CDC reported 16 chikungunya cases in travelers returning to the U.S. from an affected area.
"Emergent has achieved a major milestone as we begin our phase 3 study for our single-dose chikungunya vaccine candidate," stated Karen L. Smith, M.D., Ph.D., EVP, and chief medical officer at Emergent BioSolutions, in a press release.
"I am proud of the Emergent team for bringing us a step closer to potentially having a critical solution to address this important disease for which no vaccine or treatment is currently available."
Emergent's CHIKV VLP vaccine candidate received Breakthrough Therapy designation and Fast Track designation from the U.S. FDA in October 2020 and May 2018, respectively, and PRIME designation from the European Medicines Agency in September 2019.
These designations facilitate the development or expedite review of medicines that target an unmet medical need or demonstrate substantial improvement over available therapy.
Emergent BioSolutions is a global life sciences company located in Gaithersburg, MD, whose mission is to protect and enhance life. Through our specialty products and contract development, and manufacturing services, we are dedicated to providing solutions that address public health threats.
Our Trust Standards: Medical Advisory Committee























