Brazil Reports 65% Increase in Yellow Fever Cases

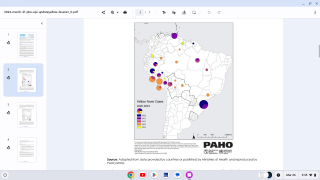
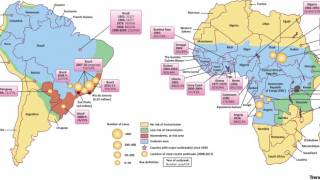
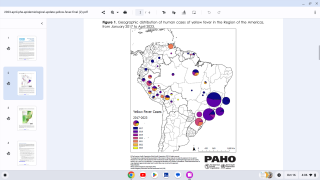
A Brazil Ministry of Health bulletin released on February 7, 2018, reported 353 confirmed cases of the yellow fever virus.
This represents a 65.8 percent increase from the previous week when 213 yellow fever cases were registered, reports Reuters.
The yellow fever death toll also increased by 17, now reaching 98 during this outbreak. This week’s Brazilian bulletin gathers data from July 2017 through January 6, 2018.
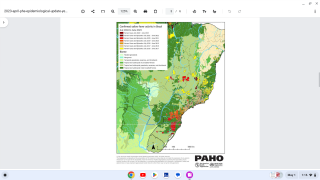
Additionally, over the past week, Sao Paulo state in Brazil recorded the most yellow fever cases, with 161, followed by the state of Minas Gerais, which reported 157 cases.
Consequently, vaccination against yellow fever is now recommended for international travelers visiting any area in the state of São Paulo.
Previously, during January 2018, Brazil announced the launch of a yellow fever vaccination campaign targeting over 19 million people in Sao Paulo, Rio de Janeiro, and Bahia states.
Brazil’s Ministry of Health reaffirmed that there has not been a confirmed case of urban yellow fever transmission. According to the health department, all the cases of yellow fever registered in Brazil since 1942 are wild, including the current ones.
This means that the current transmissions originate from the genera Haemagogus and Sabethes mosquitoes, which are located in forest environments.
These mosquitoes infect themselves from infected monkeys and then pass the yellow fever virus on to humans when they are bitten
Yellow fever virus is an acute viral hemorrhagic disease that is endemic in tropical areas. Cases can be difficult to distinguish from other viral hemorrhagic fevers such as arenavirus, hantavirus or dengue.
Symptoms of yellow fever usually appear 3 to 6 days after the bite of an infected mosquito. For most patients, these symptoms disappear after 3 to 4 days.
However, 15 percent of patients enter a second, more toxic phase, and several body systems may be affected, including the kidneys.
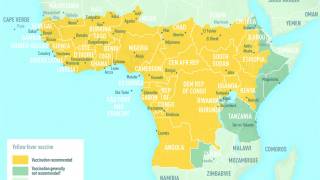
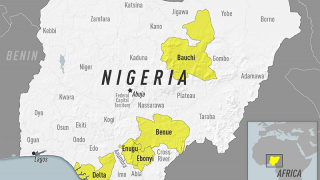
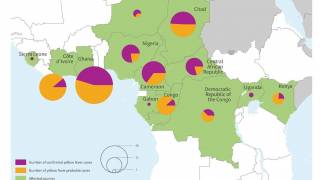
In light of the ongoing yellow fever outbreaks in Brazil, Borno, Nigeria, Peru, China, Kenya, and The Congo, the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) are reiterating the importance of persons getting vaccinated, and showing proof of yellow fever vaccination, when travelling to affected countries.
The yellow fever vaccine is recommended for people aged 9 months and older.
In some countries, travelers are not allowed to enter without a yellow fever vaccination certificate.
For most travelers, a single dose of yellow fever vaccine provides long-lasting protection. However, some travelers may require a booster dose.
Yellow fever vaccination requirements and recommendations for specific countries are available on the CDC Travelers’ Health page.
The Yellow fever vaccine is available only at designated vaccination centers in the USA, which can be found at the CDC’s Travelers’ Health Yellow Fever Vaccination Clinics page.
Sanofi Pasteur is the manufacturer of the only yellow fever vaccine (YF-Vax) licensed in the United States. Because of a depletion of the YF-Vax vaccine, Sanofi worked with the CDC to make an alternative yellow fever vaccine, 17D-204, available in the USA.
Sanofi’s Stamaril vaccine is currently available in 70 countries worldwide and has a clinical efficacy profile similar to YF-VAX.
Stamaril is available at select locations in the USA.
There are sufficient supplies of Stamaril vaccine to meet the yellow fever vaccination needs at the clinics participating in the Expanded Access Program in the US.
Talk to your healthcare provider or pharmacists to determine if you may need a dose of yellow fever vaccine or a booster dose before your trip to an area at risk for yellow fever.
Vaccines, like any medicine, can have side effects, says the CDC. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Our Trust Standards: Medical Advisory Committee
- Brazil confirms 353 yellow fever cases, including 98 deaths
- Evidence for Multiple Sylvatic Transmission Cycles During the 2016-2017 Yellow Fever Virus Outbreak, Brazil
- Federal Government of Nigeria to vaccinate 1.2 million Internally Displaced Persons (IDPs) against yellow fever in Borno State w
- This Weekly Bulletin on Outbreaks and Other Emergencies
- Brazil has a 65% increase in cases of yellow fever, according to the Ministry of Health