Yellow Fever Vaccine Coadministration Confirmed Safe for International Travelers

For international travelers to optimize an immune response from pre-departure vaccination, multiple factors such as the type of vaccine should be considered.
Another factor is the coadministration of multiple vaccines during a single appointment.
According to the U.S. CDC, most vaccines can be divided into two general categories: live or non-live.
Many live vaccines used in the USA are "live attenuated," meaning that the microbe in the vaccine is alive but has been weakened through serial passage in cultures or produced through genetic technology. Live vaccines must replicate to induce an immune response.
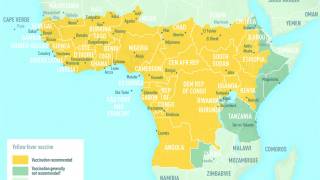
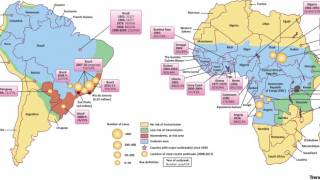
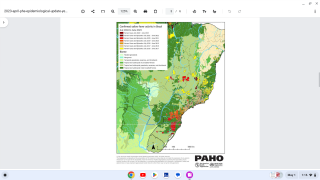
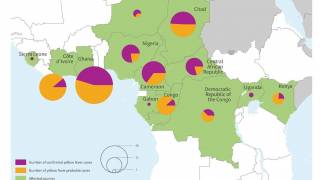
An often required live vaccine for international travel is Stamaril which protects people from the yellow fever (YF) virus.
The YF virus is a single-stranded RNA virus that transmission occurs via the bite of an infected mosquito.
A recent clinical study conducted in Europe evaluated the safety and immunogenicity following concomitant delivery of the Yellow fever virus (YFV) Stamaril® vaccine with Tick-borne encephalitis virus (TBEV) and Japanese encephalitis virus (JE) IXIARO® vaccines.
Concomitant serves to provide protection against multiple pathogens in a shorter time span, both for individuals living in or traveling to disease-endemic areas.
Published on the MedRxiv preprint server on July 11, 2022, these researchers concluded that inactivated TBEV, or IXIARO vaccines, can be coadministered with Stamaril without an increased risk of adverse events and without reduced development of neutralizing antibodies to the respective viruses.
Furthermore, yellow fever vaccines can safely be delivered in the same upper arm without adverse outcomes.
And across all study cohorts, most participants receiving the Stamaril vaccine had detectable levels of YFV NS5-RNA seven days after vaccination.
Furthermore, these findings suggest that TBEV or JEV vaccination and the Stamaril vaccine have no significant systemic effects on kidney or liver organ function. And of the adverse events reported following vaccination, the majority were mild, dominated by typical cold symptoms followed by classical reactogenicity-related responses.
The relative strength of this non-peer-reviewed study is that data were collected 'from a bona fide prospective clinical trial with the scientific and regulatory rigor inherent to this design, independent study monitoring of data, both of which contributed to data quality, and additionally low drop-out rates and minimal risk of selection bias.'
Based on these findings, 'we conclude that concomitant vaccination did not generally affect YFV replication in the study participants receiving concomitant vaccination,' stated these researchers.
"It's not surprising that coadministration of yellow fever vaccine with these other vaccines has been shown to be safe and effective," said Crockett Tidwell RPh, CDCES, Clinical Services Manager, United Supermarkets Pharmacy.
"Most vaccines can be given together without adverse consequences, but there are a few exceptions."
"That is why it is so important to seek the advice of a travel health specialist that is very familiar with these non-routine vaccines and make sure you will have the best protection possible," concluded Tidwell, a pharmacy leader in delivering yellow fever vaccine services in Lubbock, Texas.
While the Stamaril vaccine is available internationally, the U.S. FDA has approved the YF-Vax vaccine.
There are no substantial differences in the reactogenicity or immunogenicity of these products. Both of these yellow fever vaccines are produced by Sanofi Pasteur.
In the USA, certified pharmacies and clinics offer YF-Vax vaccination services, which are recommended about one month before international travel.
Importantly, following vaccination, travelers should ensure their 'Yellow Fever Card' is accurate.
The International Certificate of Vaccination and Prophylaxis proves that you recently completed a vaccination when entering a yellow fever-endemic country.
Note: The study was approved by the Stockholm Local Regional Ethical Committee (2017/1433-31/1) and the Swedish Medical Products Agency (5.1-2017-52376). It is registered in the European database (CT 2017-002137-32).
And no industry conflicts of interest were disclosed by the research team.
Vax-Before-Travel publishes fact-checked, research-based vaccine news curated for international travelers.
Updated on Oct. 30, 2022 with related links.
Our Trust Standards: Medical Advisory Committee