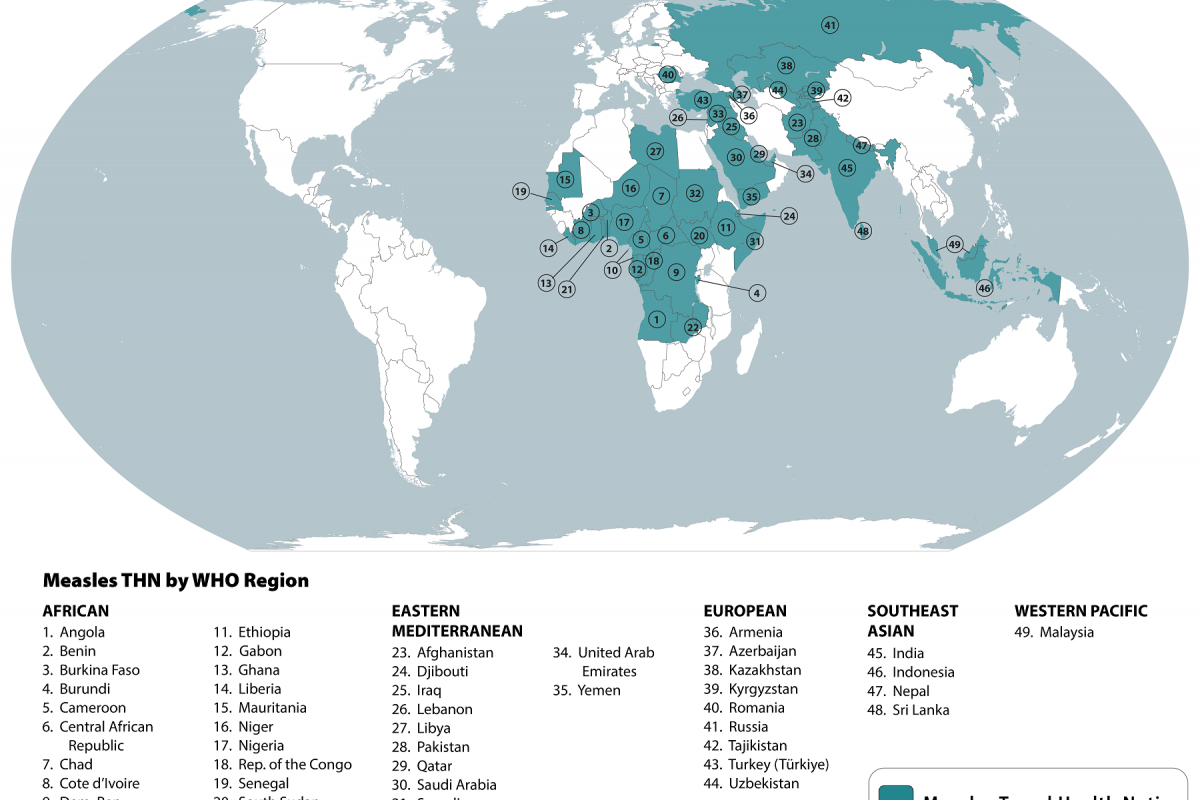
Despite the expenditure of over $3 billion to fight malaria outbreaks since 2021, the world still witnessed an estimated 600,000 malaria deaths and 247 million clinical malaria cases.
To address malaria outbreaks in Africa, new vaccines have recently been deployed.
The Mosquirix™ RTS,S/AS01E malaria vaccine (RTS,S) was introduced in 2019 by national immunization programs in Ghana, Kenya, and Malawi. Researchers aimed to address questions about feasibility and impact, as well as assess safety signals observed during the phase 3 clinical trial.
In a prospective evaluation published by The Lancet on April 4, 2024, the three primary doses were effectively deployed during the first two years of implementation of RTS,S.
There was no evidence of the safety signals that had been observed in the phase 3 trial, and the introduction of the vaccine was associated with substantial reductions in hospital admissions with severe malaria.
This evaluation continues to assess the impact of four doses of Mosquirix.
This study was funded by Gavi, the Vaccine Alliance, the Global Fund to Fight AIDS, Tuberculosis, and Malaria, and Unitaid. No industry conflicts were disclosed.
In addition to RTS,S, the R21/Matrix-M™ malaria vaccine, co-produced by scientists at the University of Oxford, Novavax AB, and Novavax Inc., has been offered in Africa in 2024.
As of April 2024, the U.S. Food and Drug Administration had not approved either malaria vaccine.