Does Breast Milk Pass mRNA to Infants
Initially, when vaccines were deployed with Messenger RNA (mRNA) encapsulated in lipid nanoparticles (LNPs), researchers thought it remained near the injection site and quickly degraded.
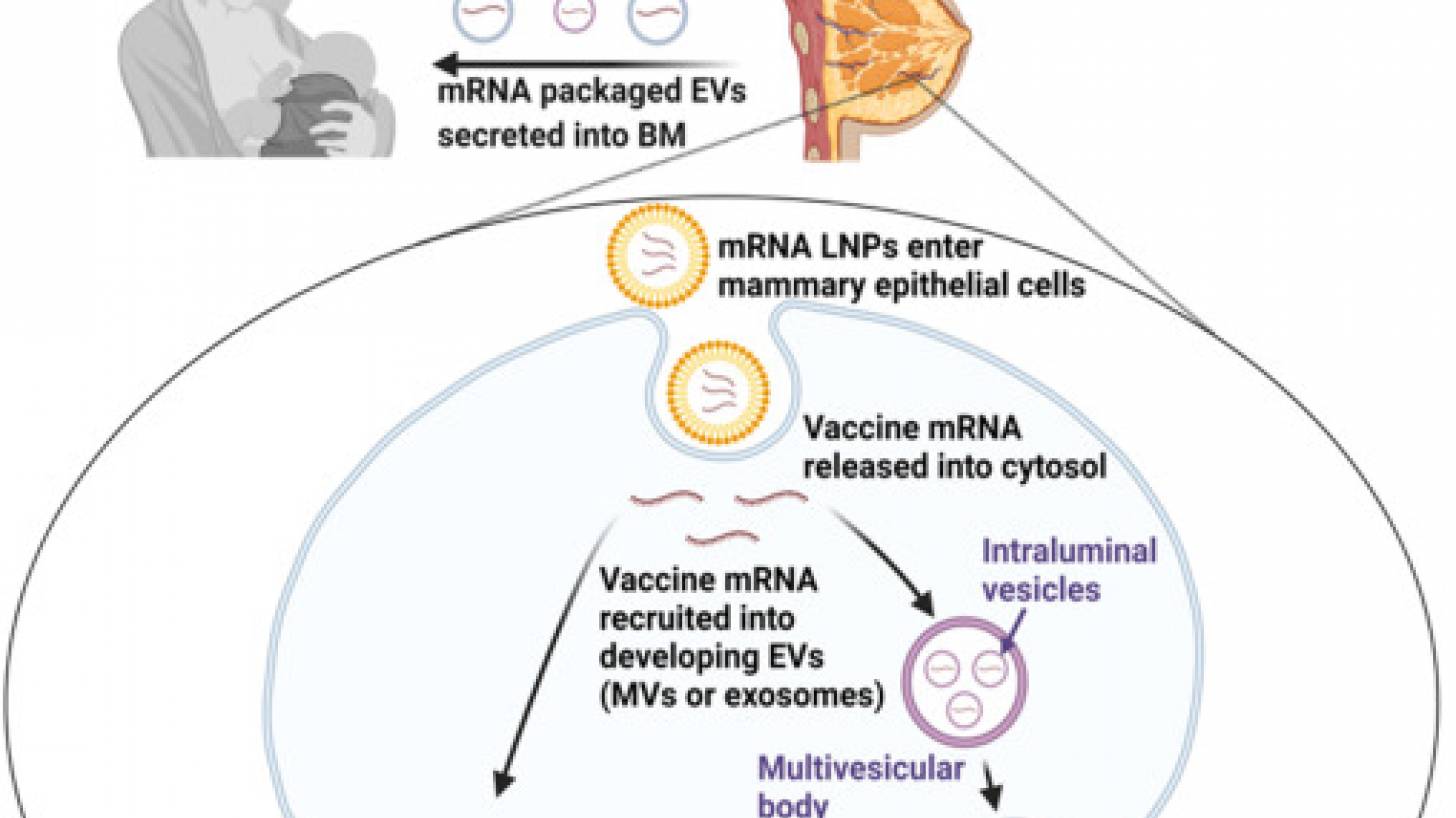
However, recent reports published by well-known clinical journals suggest that the mRNA/LNPs can enter the bloodstream, accumulate in tissues, and may be passed to infants in expressed breast milk (EBM).
To better understand how this occurrence may impact infants, a leading journal published a study supported by the Department of Pediatrics, NYU-Grossman Long Island School of Medicine.
This study reported that on September 19, 2023, of 13 vaccinated lactating women (20 exposures), trace mRNA amounts were detected in 10 women's EBM for up to 48 hours post-vaccination.
The potential bioactivity of the mRNA vaccine in EBM depends on several factors, including the concentration of biologically active mRNA and the specific cell type responding to the mRNA vaccine.
These researchers wrote, 'For the first 48 hours, when mRNA might be detected in EBM, a risk/benefit assessment is warranted.'
A separate report recently suggested that the dose of mRNA vaccine detected in EBM is of no concern because it is 0.002% of the intramuscular vaccine dose.
Another study demonstrated that a lactating mother's vaccine mRNA serum concentration was comparable to that in her EBM.
These researchers speculated that, following the vaccine administration, lipid nanoparticles containing the vaccine mRNA are carried to mammary glands via hematogenous and/or lymphatic routes.
This can be of concern in breastfed infants, considering the minimum mRNA dose needed to elicit an immune reaction in infants <6 months of age is unknown, wrote these researchers.
This study suggested a discussion between breastfeeding mothers and healthcare providers can address the benefit/risk considerations of continuing breastfeeding or withholding EBM for 48 hours after vaccination.
This opinion is consistent with the U.S. CDC's Interim Clinical Considerations of not recommending mRNA vaccine exposure in infants <6 months of age because of the lack of safety studies and until vaccine exposure risks are clinically evaluated.
Furthermore, concerns regarding vaccine exposure in EBM are not unprecedented.
For example, yellow fever and smallpox live-attenuated vaccines have been detected in EBM.
The new study's researchers concluded, 'Our findings suggest vaccine mRNA spreads systemically and can be packaged into EBM. This research should prompt a discussion among the experts responsible for formulating policies related to breastfeeding after mRNA vaccination, as information transparency is imperative as the risk/benefit balance of any vaccine can change over time.'
'Although we believe breastfeeding after mRNA vaccination is safe, a dialogue between a breastfeeding mother and her healthcare provider should address the benefit/risk considerations of breastfeeding in the first two days after maternal mRNA vaccination.'
Moreover, 'the significance of this research extends beyond the scope of the current mRNA vaccines,' concluded these researchers.
Article was updated on Sept. 22, 2023, for related links.
Our Trust Standards: Medical Advisory Committee
- JAMA 2022 - Detection of m RNA Vaccines in Human Breast Milk
- Biodistribution of mRNA COVID-19 vaccines in human breast milk
- Neutralizing Activity and SARS-CoV-2 Vaccine mRNA Persistence in Serum and Breastmilk After Vaccination in Lactating Wo
- Codominant IgG and IgA expression with minimal vaccine mRNA in milk of vaccinees