$704 Million Awarded for Advanced Ebola Treatment

Emergent BioSolutions Inc. recently announced today that it was awarded a 10-year contract by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), valued at up to a maximum of $704 million.
This contract is for advanced development, manufacturing scale-up, and procurement of Ebanga™, a U.S. FDA-licensed Ebola virus disease (EVD) treatment.
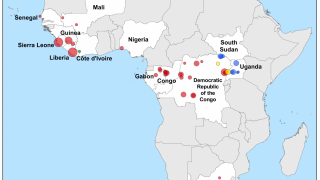
The initial EVD case first appeared in 1976, causing numerous Zaire Ebola outbreaks in African countries.
For example, local media reported on May 8, 2023. an EVD case in The Democratic Republic of Congo.
In the United States, eleven people were treated for EVD during the 2014-2016 epidemic in Africa. On September 30, 2014, the U.S. CDC confirmed the first travel-associated case of EVD diagnosed in the U.S. in a man who traveled from West Africa to Dallas, Texas. The patient died on October 8, 2014.
Since then, the U.S. Department of Homeland Security has determined that EVD threatens national health security.
"For almost 25 years, Emergent has tackled the most serious diseases to assist governments in their preparedness and response strategies," said Dr. Kelly Warfield, senior vice president at Emergent, in a press release on July 31, 2023.
"Ebola virus can emerge unexpectedly, posing a risk to global health. Its elusive nature makes it difficult to predict when and where an outbreak may occur, underscoring the importance of preparedness efforts against this public health threat."
The 10-year contract consists of a base period of performance with two option periods for advanced development valued at approximately $121 million ad option periods for procurement of Ebanga treatment over five years valued at up to $583 million.
If all option periods are exercised, the total contract value will be valued at up to approximately $704 million.
Ebanga (ansuvimab-zykl, mAb114) is a monoclonal antibody with antiviral activity provided through a single infusion and was developed for the treatment of Ebola by Ridgeback Biotherapeutics under a license from the U.S. National Institute of Allergy and Infectious Diseases.
In 2022, Emergent and Ridgeback Bio entered into a collaboration whereby Emergent is responsible for the manufacturing, sale, and distribution of Ebanga in the U.S. and Canada, and Ridgeback serves as the Global Access Partner for Ebanga treatment, ensuring it remains available to patients in endemic countries free of charge through Ridgeback's compassionate use program.
This project has been funded in whole or in part with federal funds from HHS, ASPR, and BARDA under contract number 75A50123C00037.
EVD is severe and often fatal, with case fatality rates ranging from 25% to 90%, and is transmitted via bodily fluids, zoonotic transmission, or contact with contaminated surfaces, says the World Health Organization (WHO).
The WHO recommended using monoclonal antibody treatments for Ebola infections in 2022.
Ebolavirus vaccines have been approved by the U.S. Food and Drug Administration, European Medicines Agency, the WHO, and the U.K. since 2019. As of June 2023, Zaire Ebola vaccines and antibody treatments remain in limited distribution in the U.S.
As of July 2023, no approved vaccines protect people against Sudan Ebolavirus (SUDV). However, the WHO confirmed three SUDV candidate vaccines are being tested in the Solidarity Against Ebola human clinical studies.
Our Trust Standards: Medical Advisory Committee