Lassa Fever Vaccine Candidate Progresses Into Phase 2 Research
An advanced vaccine candidate for the Lassa fever virus (LASV) today announced the start of its second phase of clinical trials.
This is a significant development, as no approved vaccines for LASV are currently available.
The trial sponsor, International AIDS Vaccine Initiative (IAVI), a nonprofit scientific research organization, confirmed in a press release on April 4, 2024, that participants at HJF Medical Research International in Nigeria had been vaccinated in the first Phase 2 clinical trial of the vaccine candidate rVSV∆G-LASV-GPC.
The IAVI C105/PREVAIL15 study began on March 4, 2024, and is expected to enroll over 600 people in Nigeria, Ghana, and Liberia, with results expected in 2025.
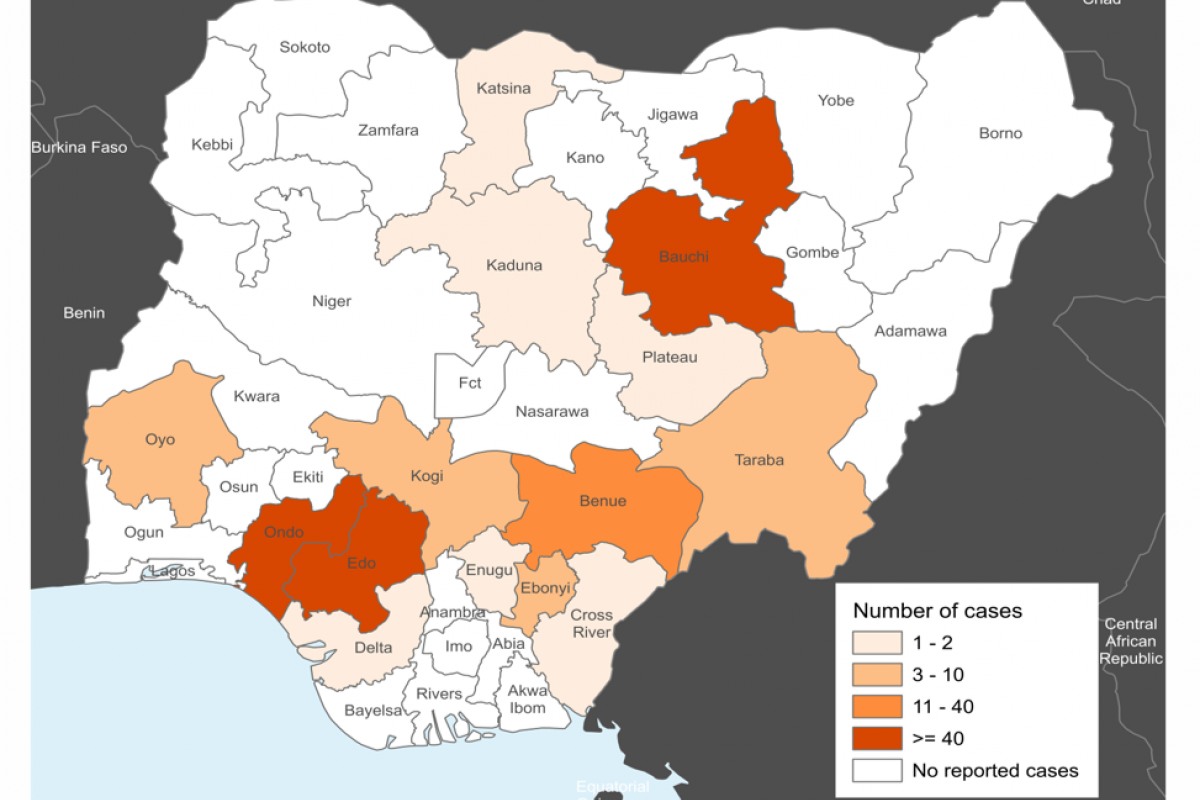
As of March 2024, 27 states in Nigeria have reported at least one confirmed case of LASV.
The vaccine has been developing since 2018 and has been supported and funded by CEPI and the European & Developing Countries Clinical Trials Partnership.
According to IAVI, rVSV∆G-LASV-GPC uses the same recombinant vesicular stomatitis virus vector platform as ERVEBO®, the single-dose Zaire ebolavirus vaccine licensed in North America, Europe, and various African countries.
“Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations – the overarching goal of IAVI’s emerging infectious disease program,” stated Swati Gupta, DrPH, MPH, vice president and head of emerging infectious diseases and epidemiology, IAVI.
The virus causes acute viral hemorrhagic illness and results in approximately 5,000 deaths and 300,000 illnesses in West Africa each year.
Furthermore, LASV has been included in the World Health Organization's R&D Blueprint of priority pathogens for which accelerated research and development and countermeasures are urgently needed.
In addition to rVSV∆G-LASV-GPC, several other LASV vaccine candidates are conducting clinical research in 2024.
Our Trust Standards: Medical Advisory Committee