Lassa Fever Vaccine
Lassa Fever Vaccine 2024
Lassa fever virus (LASV) vaccine candidates are not U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) approved as of April 2024. Given its potential to cause a public health emergency of international concern, LASV is included in the World Health Organization R&D Blueprint of priority pathogens for which there is an urgent need for accelerated research and development and countermeasures.
Lassa Fever Vaccine Candidates
As of 2024, four LF vaccine candidates (INO-4500, MV-LASV, rVSV∆G-LASV-GPC, and EBS-LASV) have entered the clinical clinical stage.
International AIDS Vaccine Initiative (IAVI) LASV vaccine candidate is conducting a Phase 2 (IAVI C105) Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Safety, Tolerability, and Immunogenicity of rVSV∆G-LASV-GPC Vaccine in Adults and Children Residing in West Africa. A Phase I (IAVI C102) clinical trial was supported by the Coalition for Epidemic Preparedness Innovations. Batavia BioScienes manufactured the IAVI's LASV vaccine candidate in Leiden, The Netherlands.
ChAdOx1-Lassa-GPC is a chimpanzee adenovirus-vectored vaccine candidate encoding the Josiah strain LASV glycoprotein precursor gene.
Themis Bioscience GmbH is a recombinant, live-attenuated, measles-vectored Lassa fever vaccine candidate (MV-LASV). In a first-in-human phase 1 trial, MV-LASV (V182-001) showed an acceptable safety and tolerability profile, and immunogenicity was unaffected by pre-existing immunity against the vector.
Inovio discontinued the development of product candidates targeting Lassa Fever (INO-4500) on November 17, 2022.
Lassa Fever Overview
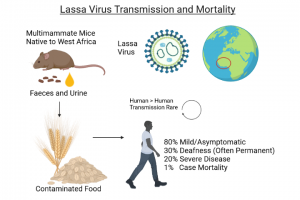
Lassa fever (LF) is an acute viral hemorrhagic fever (VHF) caused by the Lassa virus. The natural reservoir for the LASV is the Mastomys natalensis rodent (African rat). Lassa virus is endemic in the West African countries of Benin, Ghana, Guinea, Liberia, Mali, Nigeria, and Sierra Leone.
Lassa Fever Vaccine News
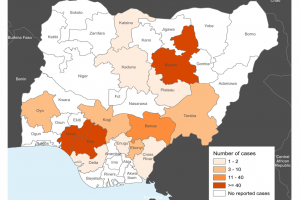
February 18, 2024 - Ebonyi Ministry of Health disclosed that Lassa Fever killed ten persons from January to mid-February 2024.
January 4, 2024 - Research findings defined a prefusion-stabilized GPC trimer, revealed an apex-situated site of vulnerability, and demonstrated that a cleavage-intermediate LASV trimer elicits LASV-neutralizing responses.
August 24, 2023 - Imunon, Inc. entered into a Cooperative Research and Development Agreement with the National Institute of Allergy and Infectious Diseases to evaluate the immunogenicity and efficacy of two IMUNON DNA-based Lassa vaccine candidates.
April 24, 2023 - The U.S. CDC issued a Watch - Level 1 Practice Usual Precautions alert regarding Nigeria's Lassa Fever outbreak.
April 18, 2023—The Nigeria Centre for Disease Control and Prevention reported that Lassa fever-related fatalities reached 151 in 2023.
March 16, 2023 - The Lancet published a study that concluded MV-LASV showed an acceptable safety and tolerability profile, and immunogenicity seemed to be unaffected by pre-existing immunity against the vector.
March 1, 2023 - The U.S. CDC issued a Watch - Level 1 Practice Usual Precautions notice regarding Nigeria's Lassa Fever outbreak.
January 30, 2023 - The Nigeria Centre for Disease Control and Prevention activated the national multisectoral Emergency Operations Centre for Lassa Fever at level 2 to coordinate and strengthen ongoing response activities in the country.
August 31, 2022 – IAVI announced that volunteers at the PREVAIL clinical trial site at Redemption Hospital in Monrovia, Liberia, have been vaccinated with IAVI's novel vaccine candidate against Lassa fever virus (LASV) in a Phase 1 clinical trial.