Pentavalent Meningococcal Vaccine Candidate for Adolescents Seeks Approval in 2023
Pfizer Inc. announced yesterday that the U.S. Food and Drug Administration (FDA) accepted for review a Biologics License Application (BLA) for its investigational pentavalent meningococcal vaccine candidate known as MenABCWY.
MenABCWY combines the components from its two licensed meningococcal vaccines, Trumenba® (meningococcal group B vaccine) and Nimenrix® (meningococcal groups A, C, W-135, and Y conjugate vaccine).
Pfizer submitted MenABCWY to prevent meningococcal disease caused by the most common serogroups in individuals 10 through 25 years of age.
The FDA's Prescription Drug User Fee Act goal is October 2023.
"The FDA's acceptance of our application for the pentavalent meningococcal vaccine candidate is an essential step toward helping protect individuals and communities against the most common types of meningococcal disease," said Annaliesa Anderson, Ph.D., SVP and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release issued on December 28, 2022.
"We believe our investigational MenABCWY vaccine, if approved and recommended, could help simplify the meningococcal vaccination schedule for adolescents and young adults and, in turn, improve vaccination rates and provide the broadest serogroup coverage of any meningococcal vaccine."
"The pentavalent vaccine candidate was well-tolerated in clinical trials, with a safety profile consistent with currently licensed meningococcal vaccines."
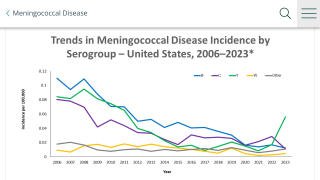
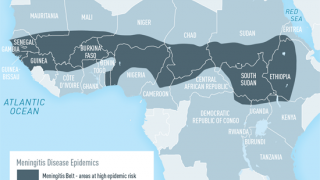
Meningococcal disease is an uncommon but serious illness that can lead to death within 24 hours and, for survivors, can result in life-altering, significant long-term disabilities, says the U.S. Centers for Disease Control and Prevention (CDC).
In the U.S., the CDC's current meningococcal vaccination platform for adolescents and young adults includes a routine recommendation for MenACWY vaccines (two doses) and a separate, shared clinical decision recommendation for MenB-specific vaccines (two doses) to achieve the broadest serogroup protection available against meningococcal disease.
According to CDC recommendations, approximately 55 million adolescents and young adults in the U.S. are in the age range for meningococcal vaccination (11-23 years old).
However, less than a third of U.S. adolescents receive even one dose of a MenB vaccine, and fewer complete the series.
Additional meningococcal vaccine information is posted at PrecisionVaccinations.com.
Our Trust Standards: Medical Advisory Committee