More Monkeypox Vaccines Coming From Michigan

The U.S. Department of Health and Human Services (HHS) confirmed it had facilitated an agreement between Bavarian Nordic and Grand River Aseptic Manufacturing (GRAM), a Michigan-based pharmaceutical contract manufacturer, to establish the first fill and finish line for the Jynneos® monkeypox / smallpox vaccine in the USA.
“We continue to do everything we can to make more vaccine doses available more quickly to those in need,” said HHS Secretary Xavier Becerra in a press release on August 18, 2022.
“Establishing a second (production) line in the U.S. will double the capacity to fill and finish these vaccines, create high-quality American jobs, and strengthen our domestic supply chain security.”
Even before the contract was signed, Bavarian Nordic initiated the technology transfer necessary for this production at GRAM, and that transfer is on track to start manufacturing later this year.
With biologics, small molecules, and vaccine capabilities, GRAM’s elite equipment and staff support pharmaceutical cGMP manufacturing, analytical testing, and regulatory filing.
To support the current monkeypox outbreak and future smallpox preparedness, the Biomedical Advance Research and Development Authority (BARDA) has ordered 5.5 million vials of Jynneos from Bavarian Nordic to be delivered from U.S. government-owned bulk vaccine stored in Denmark.
Under that procurement agreement, Bavarian Nordic agreed to complete a technology transfer allowing 2.5 million vials to be filled and finished by a U.S.-based contract manufacturer.
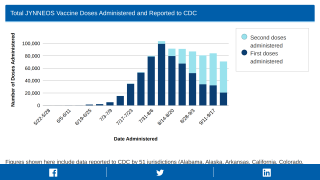
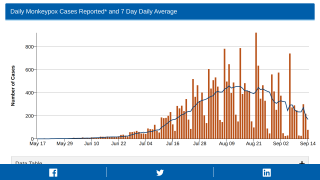
As of August 19, 2022, the U.S. government had shipped 1,061,913 Jynneos doses to states and territories, with California, Florida, and New York receiving the most vaccines.
Bavarian Nordic (BN) has already taken several steps to increase the filling capacity at its manufacturing site in Denmark, now operating at the double the capacity as before the monkeypox outbreak in May 2022, with an expectation to further increase over the coming months.
Since 2003, BN has worked with the U.S. government on developing, manufacturing, and supplying non-replicating smallpox vaccines based on replicating vaccinia virus strains.
The Company has supplied nearly 30 million doses of the vaccine to the U.S. government, with the vast majority being delivered for emergency use - and now expired - before approval of the vaccine by the U.S. FDA in 2019, which included authorization for the monkeypox indication.
The Jynneos vaccine (IMVANEX, IMVAMUNE) is based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara. It induces strong cellular activity and antibody immune response and has demonstrated an ability to stimulate a response even in individuals with pre-existing immunity against vaccinia.
Additional monkeypox vaccine development news is posted at PrecisionVaccinations.com/Monkeypox.
Note: The HHS and BN announcements were manually translated and curated for mobile readership.
Our Trust Standards: Medical Advisory Committee