Typhoid Vaccines
Typhoid Vaccines 2024
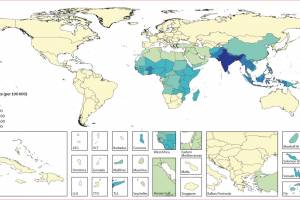
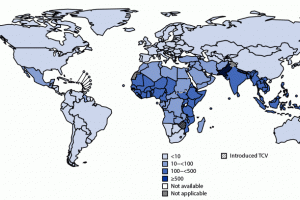
The U.S. Centers for Disease Control and Prevention (CDC) recommends typhoid vaccination for people traveling to places where typhoid fever is common, such as South Asia, especially India, Pakistan, and Bangladesh. The World Health Organization (WHO) recommended in 2018 that the typhoid conjugate vaccine (TCV) for typhoid fever control is safe and effective. The WHO recommends three types of typhoid vaccines. As of April 2024, the CDC says there are two vaccines to prevent typhoid fever. However, typhoid vaccines are not 100% effective. In February 2023, the CDC published Typhoid Fever Surveillance, Incidence Estimates, and Progress Toward Typhoid Conjugate Vaccine Introduction — Worldwide, 2018–2022. On January 25, 2024, The Lancet published - Efficacy of typhoid conjugate vaccine: final analysis. In November 2023, the Lancet Infectious Diseases published - typhoid's transformation from an environmental to a vaccine-preventable disease, 1940–2019.
Typhoid Vaccine Types
There are three types of TCV: an injectable TCV, consisting of Vi polysaccharide antigen linked to a carrier protein licensed for children from 6 months of age and adults up to 45 years or 65 years of age; an injectable unconjugated polysaccharide vaccine based on the purified Vi antigen (known as Vi-PS vaccine); and an oral live attenuated Ty21a vaccine in capsule formulation for those over six years of age. In the United States, two typhoid fever vaccines are available - oral and Injectable.
Typhoid Vaccines Authorized
Two typhoid fever vaccines are available in the United States, and the WHO currently recommends three vaccines for controlling endemic and epidemic typhoid fever.
Bharat Biotech International's Typbar TCV is a vaccine containing polysaccharides of Salmonella typhi Ty2 conjugated to Tetanus Toxoid.
Sanofi Pasteur's Typhim VI is a sterile solution prepared from the purified polysaccharide capsule of Salmonella typhi (Ty 2 strain).
Emergent BioSolutions' Vivotif oral vaccine is indicated for immunization of adults and children over six years of age against disease caused by Salmonella Typhi.
SK bioscience SKYTyphoid™ is a polysaccharide-protein conjugate vaccine developed by conjugating the polysaccharide of typhoid bacteria, which acts as the antigen, to the diphtheria toxin protein (diphtheria toxoid), which acts as a carrier. SKYTyphoid achieved WHO qualification in 2024.
Typhoid Vaccine Acceleration Consortium
The Typhoid Vaccine Acceleration Consortium (TyVAC) is a partnership between the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine, the Oxford Vaccine Group at the University of Oxford, and PATH, an international nonprofit organization. TyVAC aims to accelerate the introduction of new TCVs as part of an integrated approach to reduce the burden of typhoid in countries eligible for support from Gavi, the Vaccine Alliance. The TyVAC is funded by the Bill & Melinda Gates Foundation.
Typhoid Vaccine Effectiveness
Results from a phase 3 randomized controlled clinical trial published in The Lancet found one dose of the conjugate typhoid vaccine had an estimated efficacy of 78.3% in children ages nine months to 12 years and remained strong over four years.
Typhoid Outbreaks 2024
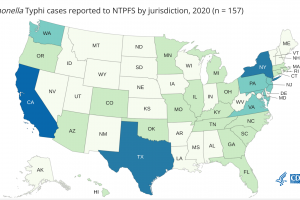
On June 16, 2023, the CDC issued a Level 1 travel advisory for an extensively drug-resistant typhoid fever outbreak. An estimated nine million people get sick from typhoid, and 110,000 people die from it every year. In the United States, about 85% of cases of typhoid fever are in undervaccinated people who arrived from other countries. About 350 people are diagnosed with typhoid fever, and 90 people are diagnosed with paratyphoid fever each year in the U.S.
Typhoid Fever Vaccine News
January 10, 2024 - The Ministry of Health and Medical Services in Fiji has confirmed a typhoid outbreak in Lau Settlement in Ra, with six cases reported from September to December, resulting in one death.
May 12, 2023 - Malawi has launched a nationwide rollout of the newest typhoid vaccine for children under 15 years of age.
September 1, 2022 - The Lancet Global Health published a study: Safety and immunogenicity of a typhoid conjugate vaccine among children aged nine months to 12 years in Malawi: a nested substudy of a double-blind, randomized controlled trial. Interpretation - This study provides evidence of TCV safety, tolerability, and immunogenicity up to 730–1035 days in Malawian children aged nine months to 12.
September 16, 2021 - Study results: Typhoid conjugate vaccines are highly effective in African children. Those children who had received TCV were 84% less likely to contract typhoid during that time.
July 29, 2022 - Review of the Recent Advances in Typhoid Vaccine Development and Challenges Ahead.