Urinary Tract Infection Vaccine Fast-Tracked

The US Food and Drug Administration (FDA) announced that it had issued Fast-Track designation for an investigational vaccine designed to treat recurrent urinary tract infections (UTIs) caused by multidrug-resistant (MDR) bacteria.
In granting Fast-Track status, the FDA believes that recurrent UTI caused by MDR bacteria is a serious condition, for which there is an immediate medical need.
This vaccine candidate is designed to create an immune response preventing bacteria from colonizing in the urinary tract, and it recently completed its first clinical trial in women.
"If approved, the vaccine could change the standard of care for recurrent UTI," said Gary Eldridge, president and CEO, Sequoia Sciences.
"Since UTI[s] are a primary source of sepsis, decreasing recurrent UTI may ultimately drive down rates of hospitalization, sepsis, and associated in-hospital mortality."
Sequoia's vaccine is designed to produce an immune response preventing bacteria from colonizing the urinary tract, and it recently completed its first clinical trial in women.
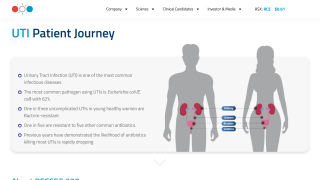
Urinary tract infections are among the most common infections in people, and antibiotic treatment is usually helpful in treating an infection. UTIs can affect several parts of the urinary tract, but the most common type of UTI is a bladder infection (also known as cystitis).
Women and girls are at a higher risk compared to men and boys because their urethra is shorter and closer to the anus, which makes it easier for bacteria to enter the urinary tract.
Each year, about 3 million patients in the United States and 10 million in North America, Europe, and Japan experience recurrent UTI, about half of which is caused by antibiotic-resistant bacteria.
Fast-track designation expedites the development and review of the vaccine through the US regulatory process.
Our Trust Standards: Medical Advisory Committee
- Sequoia Sciences Receives FDA Fast Track Designation for Vaccine for Urinary Tract Infections Caused by Multidrug-Resistant Bact
- Urinary Tract Infection
- Frequently Asked Questions: Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and NonCatheter-Associa
- Drug and Vaccine Development for the Treatment and Prevention of Urinary Tract Infections