Polio Vaccines
Polio Vaccines 2024
Two types of polio vaccines were in use in 2024, according to the U.S. Centers for Disease Control and Prevention (CDC), the European Medicines Agency (EMA), and the United Kingdom NHS. The inactivated (killed) polio vaccine (IPV) was developed by Dr. Jonas Salk in 1955 and has been offered in the United States since 2000. IVPs produce antibodies in the blood to all three poliovirus types, preventing the virus's spread. The live attenuated (weakened) oral polio vaccine (OPV) was developed by Dr. Albert Sabin in 1961. The Global Polio Eradication Initiative (GPEI) says the OPV contains a weakened strain of the poliovirus that has changed over time and now behaves similarly to wild-type polio infections. In addition, OPV can mutate sufficiently to regain virulence and lead to vaccine-derived poliovirus, which can paralyze an unvaccinated person. OPVs not only eradicated serotype 2, but they also resulted in decreased mortality rates among young children. The OPV confers cross-protection against non-poliomyelitis enteroviruses, respiratory viruses, and herpes viruses due to early activation of CD4+ and CD8+ T cells via pattern-recognition receptors, reconfiguration of innate immune cells via epigenetic manipulation, and cross-reaction between B cells and T cells, among other mechanisms.
On December 4, 2023, the U.S. CDC MMWR published updated recommendations for the use of IPV for adults known to be unvaccinated or incompletely vaccinated. Fully vaccinated adults at increased risk for poliovirus exposure may receive a single lifetime booster dose of IPV. The Global OPV Stockpile Strategy for 2022-2026 was published in 2023.
Polio Vaccines 2024
The nOPV2 vaccine produced by Bio Farma has been offered in various countries. Approximately one billion doses have been administered in more than 35 countries worldwide.
The Imovax Polio®; IPOL® IPV vaccine is indicated for active immunization of infants (as young as six weeks), children, and adults to prevent poliomyelitis caused by poliovirus types 1, 2, and 3. The IPV protects against both wild-type polio and this weakened poliovirus strain. The CDC vaccine committee reviewed Clinical Considerations for Children who Received Fractional Dose Inactivated Polio Vaccine on February 28, 2024. Refer to the ACIP IPV catch-up vaccine table for details and age groups.
Kinrix is indicated for active immunization against diphtheria, tetanus, pertussis, and poliomyelitis as the fifth dose in the IPV series in children 4 through 6.
Pediarix is a vaccine for active immunization against diphtheria, tetanus, pertussis, and infection caused by all known subtypes of hepatitis B virus and poliomyelitis.
Pentacel is a multi-vaccine that contains diphtheria, tetanus toxoids, and acellular pertussis adsorbed and inactivated poliovirus (DTaP-IPV) components and an ActHIB® vaccine component.
Quadracel vaccine is indicated for active immunization against diphtheria, tetanus, pertussis, and poliomyelitis.
Sanofi's IMOVAX-Polio IPV vaccine is manufactured by Shantha Biotechnics in Hyderabad and has been used in more than 100 countries for over 40 years. ShanIPV IPV is an inactivated polio vaccine developed by Shantha Biotechnics. It received WHO prequalification status and was produced by Sanofi in Hyderabad, India, until December 2023. Sanofi Pasteur became the first contributor of IPV to India in March 2014.
Sabin IPV, an inactivated vaccine produced by SINOVAC Biotech Ltd., is indicated for preventing the wild poliovirus and was WHO-prequalified in June 2022.
LGChem (Eupolio) is the first attenuated Sabin-IPV to obtain WHO prequalification. The main advantage is a lower biosafety risk.
SINOVAC's sIPV polio vaccine was WHO-prequalified in June 2022 to prevent poliomyelitis from infection of types I, II, and III polioviruses. sIPV is available for purchase by United Nations agencies.
Bio Farma bOPV Bivalent Type 1 & 3 Oral Poliomyelitis Vaccine.
Bilthoven Biologicals produces an inactivated polio vaccine and intends to make an OPV with Bharat Biotech.
Novel Oral Polio Vaccine Candidates
Bio Farma manufactures novel OPV candidates against polio types 1 and 3, and they are undergoing several clinical studies sponsored by PATH.
Polio Vaccinations U.S. CDC
The CDC's Advisory Committee on Immunization Practices (ACIP) Oliver Brooks, MD, FAAP, led a review of poliovirus, polio vaccination, and polio epidemiology on June 21, 2023. The ACIP's Proposed Language: Adults who have received a primary series of tOPV or IPV in any combination and are at increased risk of poliovirus exposure may receive another dose of IPV. Available data do not indicate the need for more than one-lifetime booster dose for adults with IPV. Sarah Kidd, MD, MPH, led the ACIP presentation on Adult Polio Vaccination: Recommendations for unvaccinated and incompletely vaccinated adults; Recommendations for booster doses of IPV.
Since 2000, the IPV vaccine has been available in the U.S. The CDC says the IPV can reduce how much poliovirus people shed but can't stop all virus transmission. The CDC published a poliovirus vaccine update recommending children get four doses of any combination of IPV and trivalent oral polio vaccine (tOPV) or a primary series of at least three doses of IPV or tOPV. The OPV is not offered in the U.S. Since 1961, the FDA has required testing to ensure that polio vaccines used in the United States are free of SV40 contamination.
Polio Vaccinations Africa
Since the nOPV2 vaccine launched in Africa, approximately 1 billion doses have been administered in more than 29 countries. The U.S. CDC confirmed the nOPV2 vaccine is more genetically stable and less likely to be associated with the emergence of cVDPV2.
Polio Vaccinations in The Americas
The Pan America Health Organization (PAHO) confirmed in September 2022 that polio vaccination coverage has fallen below 80% in nearly all of South America, and 12 countries (Brazil) in the region are at High or Very High risk of experiencing a polio outbreak.
Polio Vaccinations in Europe
The European Immunization Agenda 2030 is endorsed by all 53 Member States in the European Region. In 2021, only 25 of our region's 53 countries reached the 95% or over polio vaccination coverage rate that WHO recommends. In 2024, IPVs will be used in EU/EEA countries.
Polio Vaccinations Israel
In March 2023, the state of New York recommended that travelers to Israel be fully vaccinated against polio. The Israeli Ministry of Health launched Operation 2-Drops in April 2022, offering additional polio vaccinations for about two million children aged six weeks to 17 years. However, as of March 2023, about 150,000 children in Israel remain unvaccinated against poliovirus.
Polio Vaccinations United Kingdom
The U.K. Health Services Agency (UKHSA) confirmed an IPV Booster campaign was launched in London targeting children aged 1 to 9 on September 29, 2022. The polio vaccine is part of the NHS childhood vaccination schedule. It's given when children are 8, 12, and 16 weeks old as part of the 6-in-1 vaccine; then at three years and four months old as part of the 4-in-1 (DTaP/IPV) pre-school booster, and at 14 years old as part of the 3-in-1 (Td/IPV) teenage booster. In the U.K., children need all these vaccinations to be fully vaccinated against polio.
Polio Vaccine Effectiveness
Estimates of vaccine effectiveness against paralytic polio range from 36% to 89% for one dose, and IPV vaccination appears to reduce the mean quantity of shed poliovirus by 63% to 91%. Research indicates no significant difference between IPV and unvaccinated individuals in the odds of poliovirus shedding. A U.S. CDC-funded study published by The Lancet on May 10, 2023, concluded that co-administration of nOPV2 and bOPV interfered with immunogenicity for poliovirus type 2 but not for types 1 and 3. The blunted nOPV2 immunogenicity we observed would be a significant drawback of co-administration as a vaccination strategy.
Polio Vaccine Fractional Dose
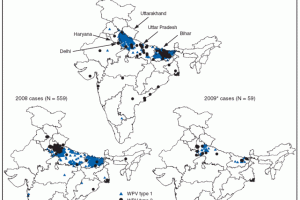
In 2016, the World Health Organization (WHO) announced a global shortage of IPV, specifically in India. In response, WHO's Strategic Advisory Group of Experts on Immunization (SAGE) recommended a strategic shift to fractional-dose inactivated poliovirus vaccine (fIPV), a smaller dose of the same vaccine, equal to 1/5 of a standard dose, says the GPEI. Studies show that two doses of fractional dose IPV administered by intradermal injection produce an even more robust immune response than a single full IPV dose.
Polio Vaccine Price
The U.S. CDC confirmed the Vaccines For Children program is a federally funded program that provides IPV vaccines and medicines at no cost to children who might not otherwise be vaccinated because of their inability to pay. The U.S. CDC Vaccine Price List was updated in 2023. This UNICEF table overviews. For people in the U.S. not covered by health insurance, a polio booster typically costs about $100. For example, pharmacies may charge about $100 for an IPV polio booster shot.
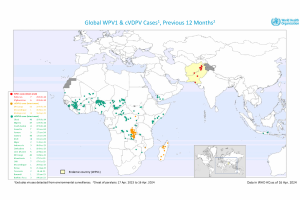
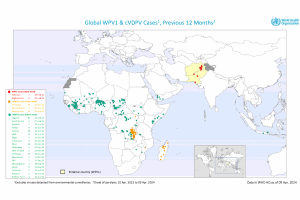
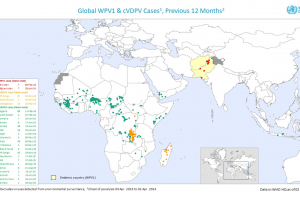
Poliovirus Outbreaks
The latest news on polio outbreaks is posted by PrecisionVaccinations.
Polio Vaccine Misinformation Management
Yale Institute for Global Health and The Public Good Projects partnered in 2020 to create the Vaccine Misinformation Management Field Guide. This guide aims to help organizations address the global infodemic by developing strategic and well-coordinated national action plans to rapidly counter vaccine misinformation and build demand for vaccinations informed by social listening.
The Digital Community Engagement (DCE) initiative recruits digital volunteers through an interactive online platform, uInfluence, to promote accurate information on polio and vaccines. In 2022, over 5 million online social listening results were analyzed from 41 countries in more than 100 languages.
uInfluence has about 75,000 digital volunteers who repurpose content shared by uInfluence on Facebook and Instagram and dispel vaccine and polio misinformation. In 2022, content posted through uInfluence channels and amplified by digital volunteers reached 74 million people.
Polio Vaccine Transition
In 2016, the CDC announced to address the risks posed by type 2 circulating vaccine-derived polioviruses, the type 2 component of the OPV was withdrawn through a switch from the tOPV) to bOPV, which contains only attenuated viruses of types 1 and 3. However, the bOPV vaccine does not offer immunity against serotype 2. This change reduced the risk of tOPV seeding new cVDPV2 outbreaks in the U.S.
The GPEI reports that before April 2016, the trivalent oral poliovirus vaccine (tOPV), which contains types 1, 2, and 3) was the predominant vaccine used for routine immunization against poliovirus. Before the development of tOPV, monovalent OPVs (mOPV2) were developed in the early 1950s but were discontinued upon the adoption of tOPV. Following April 2016, the tOPV was replaced with the bivalent oral poliovirus vaccine (bOPV). As of February 2023, the tOPV remains used with children in countries such as Somalia. On August 9, 2023, the Strategy Committee of the GPEI announced it commissioned a formal evaluation of the 2016 global withdrawal of Sabin poliovirus 2 (OPV2) and switched from tOPV to bOPV. The review aims to generate critical lessons learned from the OPV2 withdrawal to guide the direction of the GPEI, including future OPV withdrawal efforts. The finalization and publication of the evaluation are planned for mid-2024. On May 12, 2023, the CDC reported from January 2021–March 31, 2023, GPEI supported 48 countries, during which approximately 988 million bOPV, 616,000 IPV, 960,000 fractional IPV, 90 million mOPV2, 595 million nOPV2, and 100 million tOPV doses were administered. The 6th Transition Independent Monitoring Board report was published on August 2, 2023, evaluating the progress and challenges of the polio transition process and recommending strengthening work at the global, regional, and country levels.