Paxlovid Rebound Cases Confirmed by CDC

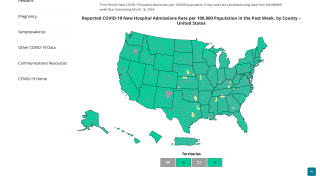
The U.S. Centers for Disease Control and Prevention (CDC) announced today the potential for recurrence of COVID-19 or “COVID-19 Rebound” following treatment with the oral antiviral Paxlovid.
The CDC says, ‘Recent case reports document that some patients with normal immune response who have completed a 5-day course of the oral antiviral Paxlovid for laboratory-confirmed infection and have recovered can experience recurrent illness 2 to 8 days later, including patients who had been vaccinated and/or boosted.’
The Health Alert Network Health Advisory CDCHAN-00467 was published on May 24, 2022, to update healthcare providers, public health departments, and the public on the recommended early-stage treatment of mild to moderate COVID-19 among persons at high risk for progression to severe disease.
A brief return of symptoms may be part of the natural history of SARS-CoV-2 (the virus that causes COVID-19) infection in some persons, independent of treatment with Paxlovid and regardless of vaccination status.
There is limited information currently available from case reports that suggest that persons treated with Paxlovid who experience COVID-19 rebound have had a mild illness; there are no reports of severe disease.
However, possible transmission of infection during the COVID-19 rebound has been recently described; and it remains unknown whether transmission during rebound differs from the likelihood of transmission during the initial infection.
Regardless of whether the patient has been treated with an antiviral agent, the risk of transmission during COVID-19 rebound can be managed by following CDC’s guidance on isolation.
People with recurrence of COVID-19 symptoms or a new positive viral test after testing negative should restart isolation and isolate again for at least five days. And some people may continue to test positive after day ten but are considerably less likely to shed infectious coronavirus.
The U.S. FDA approved Paxlovid (nirmatrelvir tablets; ritonavir tablets) to reduce the risk of hospitalization and death for patients with mild-to-moderate COVID-19 who are at risk of disease progression and severe illness.
In the Paxlovid clinical trial, a small number of participants had one or more positive SARS-CoV-2 RT-PCR test results after testing negative or an increase in the amount of SARS-CoV-2 detected by PCR after completing their treatment course.
This finding was observed in persons administered Paxlovid and in persons given a placebo.
CoronavirusToday publishes fact-checked research-based news.
Our Trust Standards: Medical Advisory Committee