$46.3 Million Award for More Bird Flu Vaccines

CSL Seqirus today announced it was selected by the Biomedical Advanced Research and Development Authority (BARDA) to deliver one bulk lot of H5N8 A/Astrakhan antigen to the U.S. government.
This acquisition of a bulk lot will increase BARDA's stockpile of vaccines to support rapid response in an associated influenza pandemic.
CSL Seqirus has been working with BARDA in a longstanding partnership for over a decade, including numerous R&D and manufacturing activities and awards supporting BARDA's pandemic preparedness objectives.
Confirmed on August 28, 2023, this is the third award CSL Seqirus has received from BARDA in the last two years related to the ongoing outbreak of HPAI in the United States.
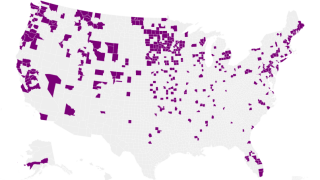
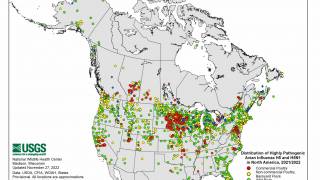
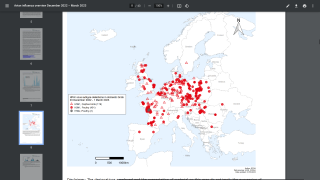
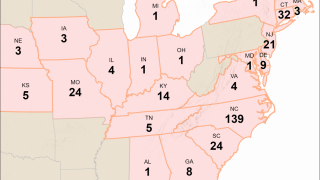
As of August 2023, the Pan American Health Organization reported H5N1 viruses (clade 2.3.4.4b) had been detected in 15 countries in Latin America, the Caribbean, the United States, and Canada over the past 18 months.
This award follows the February 2022 award to produce an H5N8 A/Astrakhan virus vaccine seed and the subsequent October 2022 announcement of the selection of CSL Seqirus to deliver an H5N8 A/Astrakhan virus vaccine candidate for assessment in a Phase 2 clinical study.
While the likelihood of sustained human-to-human transmission of bird flu is "low," according to the U.S. Centers for Disease Control and Prevention (CDC) and World Health Organization, there have been reported a small number of human cases of avian influenza A(H5), including one in the U.S. in April 2022, a case in Ecuador in January 20235 and Chile in March 2023.
"While human cases are rare, sporadic, and isolated, consistent detection of bird and mammalian cases demands vigilance," commented Marc Lacey, Executive Director, Pandemic Response Solutions, CSL Seqirus, in a press release.
"Ongoing surveillance and preparedness efforts are critical to minimize the public health risk."
CSL Seqirus used its cell-based influenza vaccine technology, as utilized for FDA-approved AUDENZ™ (Influenza A(H5N1) Monovalent Vaccine, Adjuvanted), to manufacture the H5N8 A/Astrakhan bulk vaccine at the company's Holly Springs, North Carolina, facility, which was built in partnership with BARDA.
CSL Seqirus has established and will maintain the required pandemic readiness to deliver 150 million doses of cell-based pandemic influenza vaccine within six months of an influenza pandemic declaration in the U.S.
This $46.3 million project has been supported in whole or in part with federal funds from the Department of Health and Human Services, Administration for Strategic Preparedness and Response; BARDA, under contract number 75A50122D00004.
Our Trust Standards: Medical Advisory Committee