U.S. FDA Vaccine Committee Infant and Children Authorization Presentations Released

The U.S. FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) will discuss during a digital meeting on June 15, 2022, whether, based on the totality of scientific evidence available, the benefits of the Pfizer-BioNTech COVID-19 Vaccine, when administered as a three-dose primary series, outweigh its risks for use in infants and children six months through 4 years of age.
The available data "do not suggest any new safety concerns," the FDA staff report indicates.
On June 14, 2022, under Topic 1, the committee will meet in an open session to discuss amending the EUA of the Moderna COVID-19 mRNA vaccine to include the administration of the primary series to children and adolescents 6 years through 17 years of age.
On June 15, 2022, under Topic II, the committee will meet in an open session to discuss amending the EUA of the Moderna COVID-19 mRNA vaccine to include the administration of the primary series to infants and children 6 months through 5 years of age.
And also to discuss amending the EUA of the Pfizer-BioNTech COVID-19 mRNA vaccine to include the administration of the primary series to infants and children 6 months through 4 years of age.
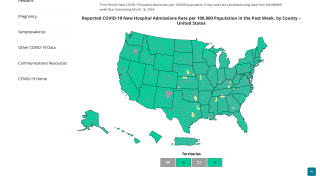
Of the total COVID-19 cases reported in the USA to date, 3.3% occurred among children 0-4 years of age.
The most common underlying medical conditions among hospitalized children were obesity (31.9%), neurologic disorders (14.8%), and asthma (14.5%).
According to death certificate data, 202 deaths have been attributed to COVID-19 among children six months to 4 years of age through May 11, 2022.
The presentations for this VRBPAC meeting are available at this FDA link.
Our Trust Standards: Medical Advisory Committee