Project NextGen
U.S. Project NextGen For Vaccines and Therapeutics 2024
Project NextGen is a $5 billion multi-government agency initiative to develop the next generation of vaccines and therapeutics. In 2023, the U.S. government (Biomedical Advanced Research and Development Authority (BARDA) announced three next-generation vaccine selections that are distinct, targeting more robust, broader, or longer-lasting immune responses. Intranasal vaccines have the potential to stop viruses at the site of infection, and self-amplifying mRNA and additional antigens may generate a more robust immune response than current vaccine technologies.
The BARDA awards support vaccine candidates for clinical evaluation:
$8.5 million to CastleVax for a vector-based intranasal vaccine candidate.
$10 million to Codagenix for a live-attenuated intranasal vaccine candidate (phase 3).
$10 million to Gritstone Bio for a self-amplifying mRNA vaccine candidate.
$9.27 million to Vaxart's Phase 2b clinical study evaluating oral pill XBB COVID-19 vaccine candidate.
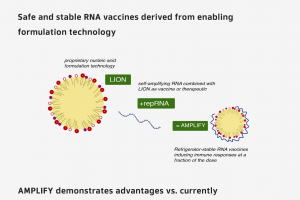
The $749,000 BARDA contract supports proof-of-concept studies for on-demand manufacturing and release processes that use HDT Bio's LION™ formulation for RNA vaccine production. LION is a proprietary nanoemulsion tailored for RNA-based vaccines and therapeutics production. It is designed to enable facile formulation and faster manufacturing compared to the characteristics of standard lipid nanoparticles used in existing products.
Project NextGen News
March 14, 2024 - The Imperial College London is leading an international consortium on a $57 million project to develop advanced virus-blocking coronavirus vaccines.
January 19, 2024 - Dr. Michael Finney, Vaxart's Interim CEO, said, "We believe our oral pill vaccine platform may ultimately hold the promise of revolutionizing how we fight pandemics and vaccinate against several infectious diseases."
January 4, 2023 - ATCC announced a five-year, $87 million award from the BARDA to support the development of next-generation medical countermeasures to protect Americans from public health security threats such as coronaviruses.
November 2, 2023 - NIH/NIAID Project NextGen selected Tonix Pharmaceuticals' TNX-1800 for Inclusion in vaccine studies.
October 13, 2023 - The U.S. Department of Health and Human Services announced the selection of initial next-generation vaccine candidates and more than $500 million in awards for Project NextGen – kick-starting planning for Phase 2b clinical trials and technologies that advance innovative next-generation vaccine and therapeutics platforms.
October 10, 2023 - Ocugen, Inc. announced that the NIAID will conduct a trial comparing the administration of Ocugen's mucosal vaccine candidate, OCU500, via two different mucosal routes: inhalation into the lungs and as a nasal spray. With funding from Project NextGen, NIAID will cover the total cost of the clinical trials, including operations and related analysis.
August 10, 2023 - The Institute for Progress filed the following comment in response to BARDA's Request For Information on ways Project NextGen could ensure future COVID-19 vaccine technologies bolster preparedness against future variants.
September 26, 2023 - The U.K. Health Security Agency agreed to an advance purchase agreement with CSL Seqirus to be on standby to produce over 100 million influenza pandemic vaccines if or when needed in the U.S.
September 22, 2023 - The U.S. CDC announced the recipients of 13 funding awards to establish a first-of-its-kind national network, the Outbreak Analytics and Disease Modeling Network (OADMN). The awards, totaling $262.5 million in funding over five years, will support state and local decision-makers in developing and implementing new tools to detect, respond to, and mitigate public health emergencies more effectively.
September 13, 2023 - ICON plc announced it is partnering with BARDA, contract number 75A50120D00017, to execute a clinical trial to evaluate the effectiveness of next-generation COVID-19 vaccine candidates.
March 15, 2021 - The Military Health System and Defense Health Agency granted the $72 million CDMRP PRMRP Technology/Therapeutic Development Award, a product-driven award mechanism intended to provide support for the translation of promising preclinical findings into products for clinical applications, including prevention, detection, diagnosis, treatment, or quality of life, in at least one of the Congressionally directed FY21 PRMRP Topic Areas.
Disease X information is posted at Precision Vax.