MERS Vaccines
Middle East Respiratory Syndrome (MERS) Vaccines 2024
The U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), the World Health Organization (WHO), and the Kingdom of Saudi Arabia have not approved a Middle East Respiratory Syndrome Coronavirus (MERS-CoV) vaccine candidate as of April 2024. Still, several vaccine candidates are conducting clinical trials. Efforts to develop an effective and safe human MERS-CoV vaccine have progressed, with a few vaccine candidates having reached human studies; these vaccines are based on DNA platforms, viral vector platforms, and modified vaccinia Ankara. On April 10, 2023, the U.S. government announced Project-NextGen, which intends to empower companies to expedite the development of vaccines and therapies for coronaviruses such as MERS.
MERS-COV Vaccine Candidates
Oxford University's Pandemic Sciences Institute and the developer of the ChAdOx1 MERS vaccine announced on September 15, 2023, that eighty-four people aged 50 to 70 will participate in a study in Liverpool. This study follows two previous Phase I clinical trials in the U.K. and Saudi Arabia.
MVA MERS-S (Modified Vaccinia virus Ankara) is a vaccine candidate that contains the full-length spike gene of MERS-CoV. Vaccination with MVA-MERS-S had a favorable safety profile without severe adverse events. Homologous prime-boost immunization induced humoral and cell-mediated responses against MERS-CoV. In June 2023, the U.S. CDC reported after a single modified vaccinia virus Ankara-MERS-S vaccine; seropositive camels showed increased levels of MERS-CoV‒specific T cells and antibodies, indicating the suitability of camel vaccinations in disease-endemic areas as a promising approach to control infection.
BVRS-GamVac-Combi is conducting phase 1/2 clinical studies sponsored by the Gamaleya Research Institute of Epidemiology and Microbiology, which is part of the Health Ministry of the Russian Federation.
VTP-500 (ChAdOx1) MERS-CoV is a vaccine candidate from the University of Oxford that consists of the replication-deficient simian adenovirus vector ChAdOx1 MERS Spike protein antigen. The VTP-500 vaccine is administered as a single administration and with a homologous prime booster. CEPI is funding up to $34.8 million to develop and stockpile VTP-500 vaccines.
The inactivated rabies vectored SARS-CoV-2 S1 vaccine CORAVAX is adjuvanted with MPLA-AddaVax, a TRL4 agonist, induced high levels of neutralizing antibodies and generated a strong Th1-biased immune response.
Avacc 101 vaccine candidate is designed to provide broad protection against SARS-CoV-1, SAR-CoV-2, and MERS-CoV.
Novavax's MERS investigational vaccine was paused at the pre-clinical process.
Ralph Baric's lab at the University of North Carolina at Chapel Hill, the office of the U.S. NIAID, negotiated an agreement to develop a MERS mRNA vaccine candidate.
MERS Outbreaks
The U.S. Centers for Disease Control and Prevention (CDC) says Middle East respiratory syndrome coronavirus (MERS-CoV) is a viral respiratory infection. The zoonotic source of this virus remains unknown. The U.S. CDC's Emerging Infectious Diseases published a study (Volume 30, Number 3) in March 2024 that identified more than three case clusters over three weeks in camels from different areas of Nairobi, Kenya, and a 15% infection rate in slaughterhouse workers. In February 2024, the WHO reported that between August 2023 and February 2024, the Ministry of Health of the Kingdom of Saudi Arabia reported four MERS-CoV cases, including two deaths.
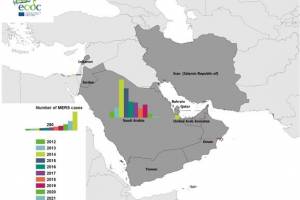
In May 2023, The Lancet Global Health published a mathematical modeling study by Daniel Laydon and colleagues that supports the importance of an efficient preparedness plan, including vaccinations, for future MERS-CoV outbreaks. A study published by the International Journal of Infectious Diseases on March 29, 2023, confirms MERS-CoV remains a threat to global health security. as variants continue circulating in humans and camels. In addition, recent recombination rates indicate coinfections with different MERS-CoV Clades: clade B (n=462), A (n=10), and C (n=5). A study published by the International Journal of Infectious Diseases on March 29, 2023, confirms MERS-CoV remains a threat to global health security. as variants continue circulating in humans and camels. In addition, recent recombination rates indicate coinfections with different MERS-CoV Clades: clade B (n=462), A (n=10), and C (n=5).
Since the first report of MERS-CoV infection in a patient with pneumonia who died in a Jeddah hospital in Saudi Arabia in 2012, about 2,605 cases with 945 associated fatalities (36%) have been reported from 27 countries in six World Health Organization.